Lipidosome nano medicine delivery system as well as preparation method and application thereof
A delivery system and nano-drug technology, applied in liposome delivery, drug combination, pharmaceutical formulation, etc., can solve the problems of limited imaging half-life, plaque rupture, large space occupation, etc., and achieve the effect of improving drug enrichment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
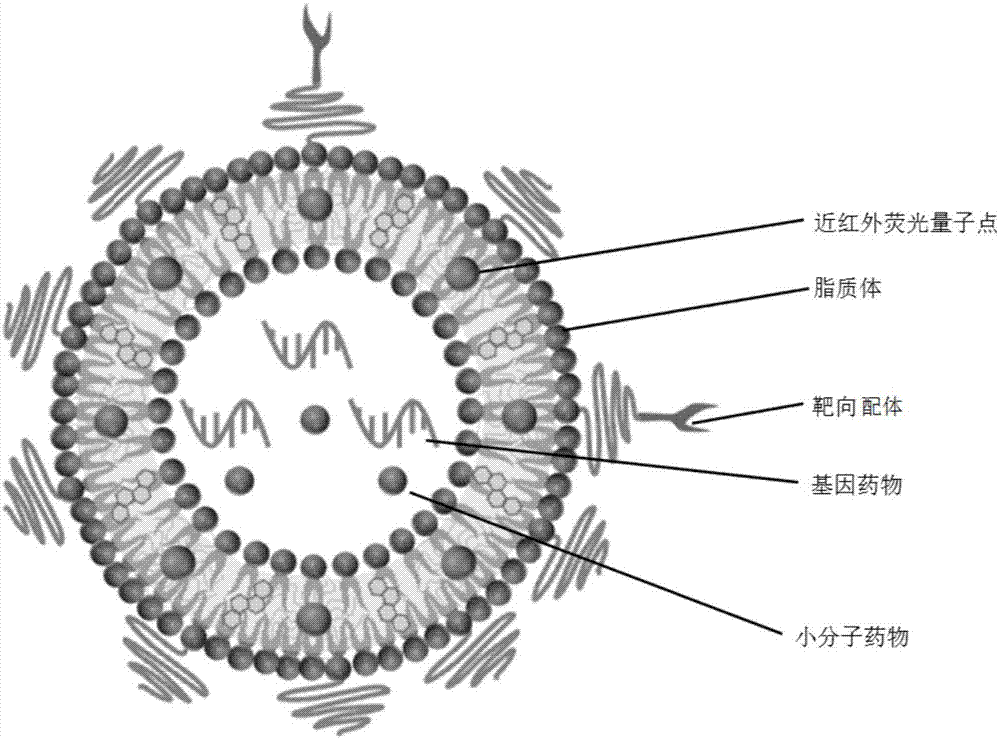
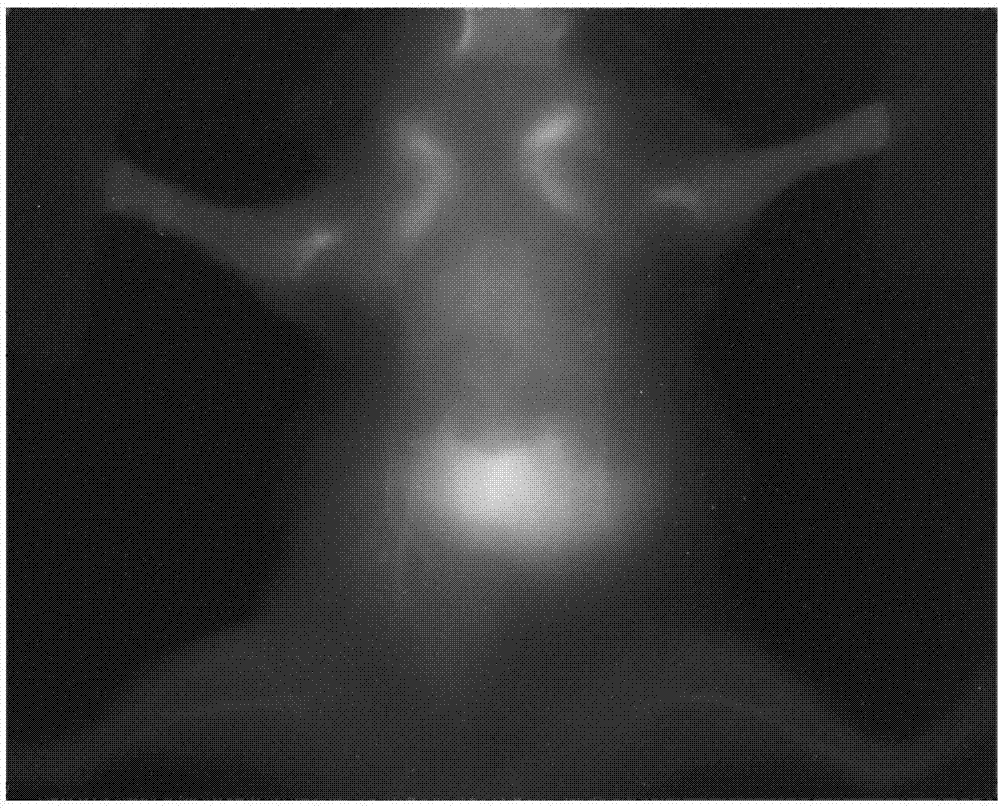
[0048] Near-infrared Ag Modified by Tumor Vascular Targeting Peptide 2 Preparation of S quantum dot liposome nano drug delivery system:
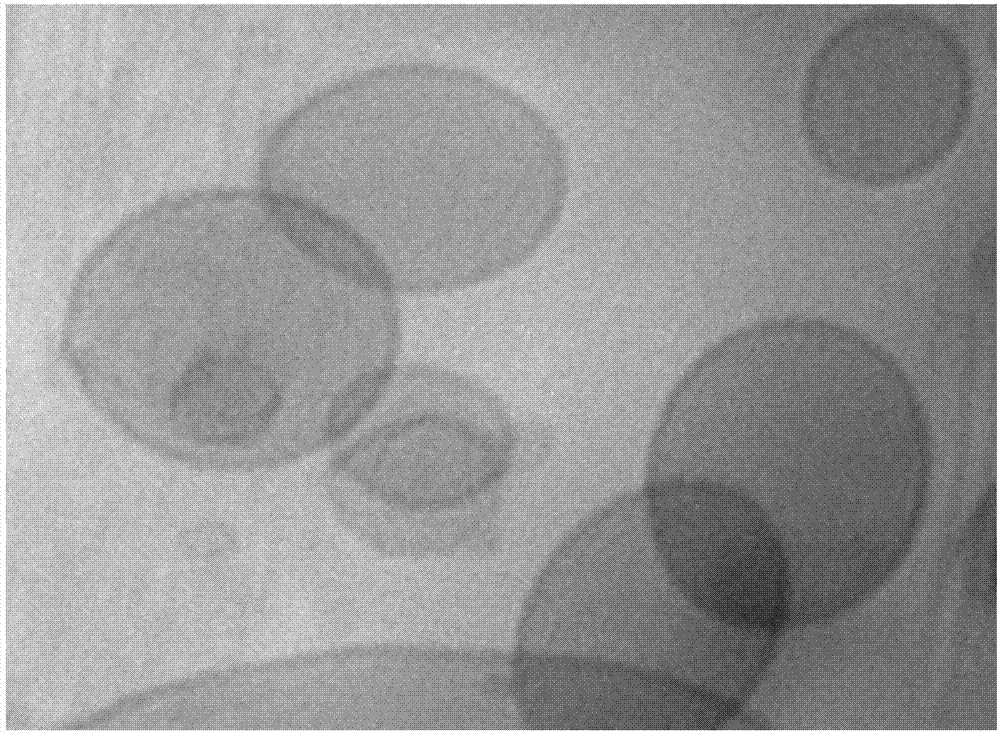
[0049] The liposome raw material component is hydrogenated soybean phospholipid (HSPC) / cholesterol (Chol) / distearoylphosphatidylethanolamine-polyethylene glycol complex (DSPE-mPEG) (45:45:2, molar ratio), tumor Vascular targeting peptide modified RGD distearoylphosphatidylethanolamine-polyethylene glycol complex HSPC / Chol / DSPE-mPEG / DSPE-mPEG-RGD (45:45:2:1, molar ratio). The above components were combined with dodecanethiol-modified near-infrared silver sulfide quantum dots (DT-Ag 2 S) Fully dissolve in chloroform, remove the organic solvent by rotary evaporation, add physiological saline to the lipid film, and rotate and shake in a water bath at 55°C to obtain Ag 2 S quantum dot liposome suspension. Further heating to 60° C., extruding through the membrane with a 200 nm carbonate membrane to obtain a liposome nano drug delivery system wi...
Embodiment 2
[0055] Near-infrared Ag Modified by Atherosclerotic Vulnerable Plaque Targeting Peptide VHPK 2 Preparation of Se quantum dot liposome nano drug delivery system:
[0056] The liposome raw material component is hydrogenated soybean phospholipid (HSPC) / cholesterol (Chol) / distearoylphosphatidylethanolamine-polyethylene glycol complex (DSPE-mPEG) (50:50:2, molar ratio), arterial Atheroma Vulnerable Plaque Targeting Peptide VHPK Modified Distearoylphosphatidylethanolamine-Polyethylene Glycol Complex HSPC / Chol / DSPE-mPEG / DSPE-mPEG-RGD (50:50:2:0.5, mol Compare). The above-mentioned components were fully dissolved in chloroform, and the organic solvent was removed by rotary evaporation, and silver selenide quantum dots (PEG-Ag-Ag) containing polyethylene glycol modified 2 The physiological saline of Se) was added to the lipid film, and rotated and oscillated in a water bath at 55°C to obtain Ag 2 Se quantum dot liposome suspension. Further heating to 60° C., extruding through the m...
Embodiment 3
[0062] Preparation of folic acid (FA)-modified near-infrared InAs quantum dot liposome nano-drug delivery system:
[0063] The liposome raw material components are hydrogenated soybean phospholipids (HSPC) / cholesterol (Chol) / distearoylphosphatidylethanolamine-polyethylene glycol complex (DSPE-mPEG) (60:55:1, molar ratio), folic acid Modified distearoylphosphatidylethanolamine-polyethylene glycol complex HSPC / Chol / DSPE-mPEG / DSPE-mPEG-RGD (60:55:1:0.5, molar ratio). The above components were fully dissolved in chloroform, and the organic solvent was removed by rotary evaporation, and the physiological saline containing polyethylene glycol-modified indium arsenide quantum dots (PEG-InAs) was added to the lipid film, and rotated and oscillated in a 55°C water bath , to obtain InAs quantum dot liposome suspension. Further heating to 60° C., extruding through the membrane with a 200 nm carbonate membrane to obtain a liposome nano drug delivery system with uniform particle size.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com