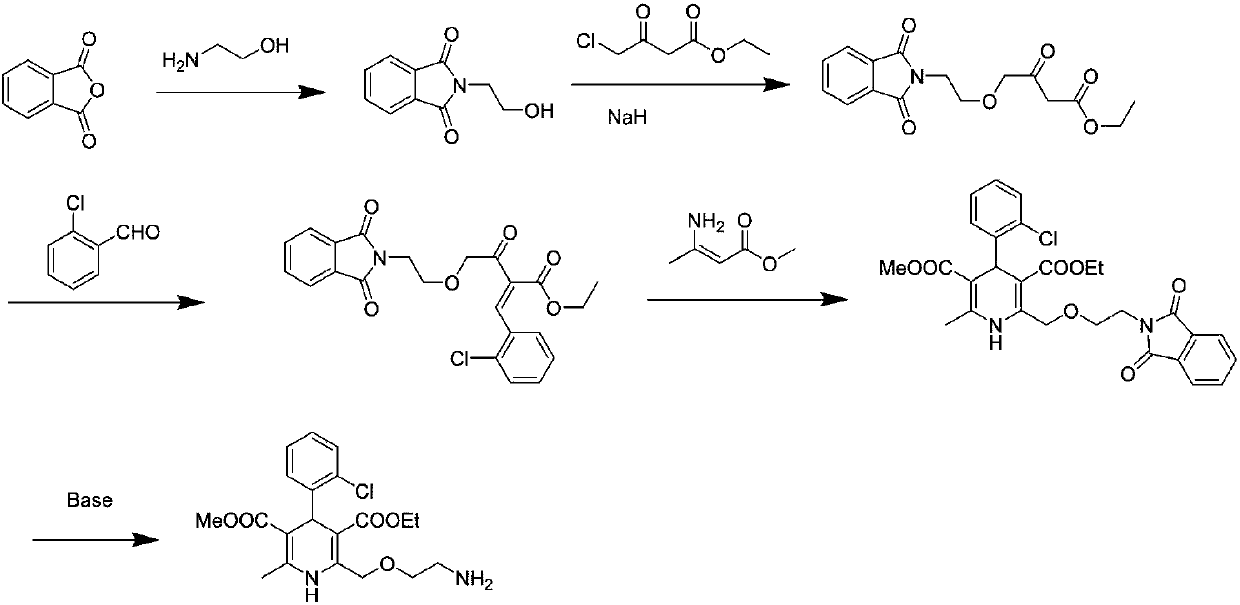
Preparation process of amlodipine intermediate
A technology for amlodipine and intermediates, which is applied in the field of preparation of amlodipine intermediates, can solve the problems of not obtaining target products and unsatisfactory results, and achieve the effects of reducing conditions and improving production safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
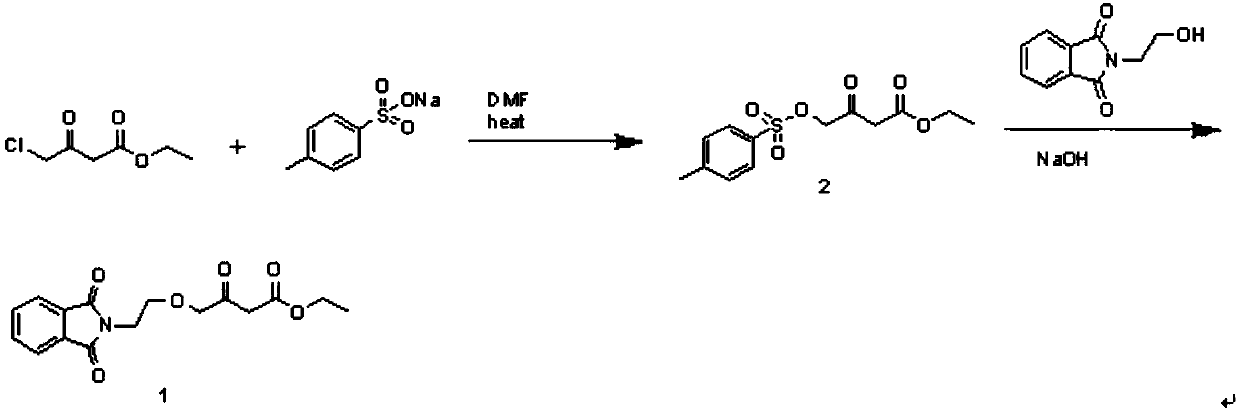
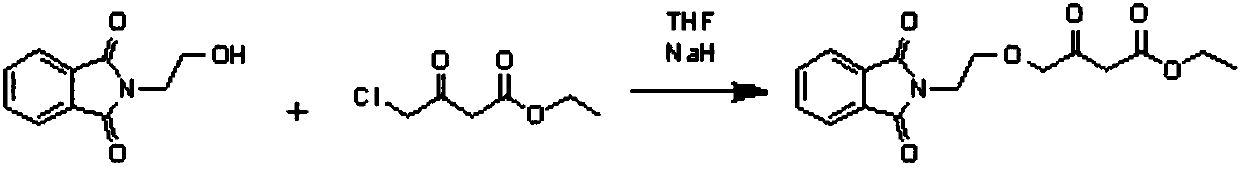
[0043] Embodiment 1: the preparation of compound 2
[0044] 350ml of DMF, 49.5g of ethyl 4-chloroacetoacetate and 67.9g of sodium p-toluenesulfonate were added to the flask, and the temperature was raised to 105°C for 5.5h. About 2 / 3 of the solvent was distilled off under reduced pressure to obtain a solid-liquid mixture. Cool to room temperature, add 500ml of water and stir for 1h. Suction filtration and washing with water gave light brown solid. It was directly used in the second reaction without purification. Yield: 88.5%.
Embodiment 2
[0045] Embodiment 2: the preparation of compound 2
[0046] 350ml of DMF, 49.5g of ethyl 4-chloroacetoacetate and 67.9g of sodium p-toluenesulfonate were added to the flask, and the temperature was raised to 105°C for 5.5h. After cooling to room temperature, the reactant was poured into 1000ml of water, stirred for 1 hour, filtered with suction and washed with water to obtain a light brown solid. It was directly used in the second reaction without purification. Yield: 86.1%.
Embodiment 3
[0048] In the comparative experiment of the preparation of compound 2, the effect of replacing sodium p-toluenesulfonate with p-toluenesulfonic acid and triethylamine on the reaction was investigated.
[0049] Add 350ml of DMF, 49.5g of ethyl 4-chloroacetoacetate and 60.2g of p-toluenesulfonic acid into the flask, and cool to 5-10°C. Slowly add 35.4 g of triethylamine dropwise, keep warm for 1 h after the addition, and then raise the temperature to 105° C. for 5.5 h. About 2 / 3 of the solvent was distilled off under reduced pressure to obtain a solid-liquid mixture. Cool to room temperature, add 500ml of water and stir for 1h. There was oil sticking to the bottle wall, poured off the water layer, added 40ml of ethyl acetate and stirred overnight. Suction filtration and washing with water gave light brown solid. Yield: 26.7%.
[0050] It can be seen from the yield that the yield of compound 2 prepared by adding p-toluenesulfonic acid and triethylamine to ethyl 4-chloroacetoa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com