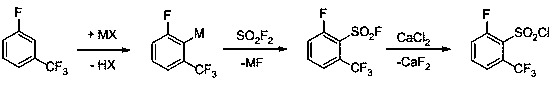
Preparation method of 2-fluoro-6-trifluoromethylbenzenesulfonylchloride
A technology of trifluoromethylbenzenesulfonyl chloride and trifluorotoluene, which is applied in the field of organic synthesis and can solve problems such as odor, high process risk, and high risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 150 mL of tetrahydrofuran and 16.4 g (0.1 mol) of m-fluorobenzotrifluoride into a 500 mL three-necked flask, cool down to -70°C, and slowly add 52 mL of 2M n-butyllithium n-hexane solution dropwise under nitrogen protection. Control the rate of addition so that the temperature of the reaction solution is not higher than -50°C. After dropping, the reaction system was evacuated to -0.09Mpa, and sulfuryl fluoride gas was introduced, and white precipitates gradually appeared in the reaction system. The sulfuryl fluoride gas was fed continuously until 11 g of gas was fed and stopped. Keep the temperature of the reaction system at -40~-20°C for 4 hours, add 2 mL of water to quench the reaction, and stir for 15 minutes. The white solid of lithium fluoride generated by separation was filtered off, and the filtrate was distilled off under normal pressure to remove n-hexane and tetrahydrofuran. Add 100mL dichloromethane to the distilled raffinate, add 15g calcium chloride i...
Embodiment 2
[0021] Add 400mL tetrahydrofuran and 49.2g (0.3mol) m-fluorobenzotrifluoride to a 1000mL stainless steel kettle, cool down to -60°C, and slowly add 160mL of 2M n-butyllithium n-hexane solution dropwise under nitrogen protection. Control the rate of addition so that the temperature of the reaction solution is not higher than -30°C. After dripping, the reaction system was evacuated to -0.095Mpa, 46g of sulfuryl fluoride gas was introduced, and the temperature of the reaction system was kept at -30°C for 6 hours, and 4mL of water was added to quench the reaction, and stirred for 15 minutes. The white solid of lithium fluoride generated by separation was filtered off, and the filtrate was distilled to remove n-hexane and dioxane. Add 300mL chloroform to the distilled raffinate, add 50g calcium chloride in 300mL water solution, stir at 60°C for reaction, white precipitate gradually precipitates in the mixture, stop the reaction for 2 hours, filter and separate the liquid, and use 1...
Embodiment 3
[0023] 150 mL of dioxane and 16.4 g (0.1 mol) of m-fluorobenzotrifluoride were added to a 500 mL stainless steel kettle, the temperature was lowered to -80°C, and 55 mL of 2M n-butyllithium n-hexane solution was slowly added dropwise under nitrogen protection. Control the rate of addition so that the temperature of the reaction solution is not higher than -50°C. After dropping, the reaction system was evacuated to -0.095Mpa, and 15g of sulfuryl fluoride gas was introduced, and the system was naturally heated to 0°C, and the temperature of the reaction system was kept at 0-10°C for 3 hours, and 0.5mL of water was added to quench the reaction, and stirred 15 minutes. The white solid of lithium fluoride generated by separation was filtered off, and the filtrate was distilled to remove n-hexane and dioxane. Add 100mL of dichloromethane to the distilled raffinate, add 18g of calcium chloride in 100mL of water, stir at room temperature, a white precipitate gradually precipitates in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com