Nanometer medicine for controlled photodynamic treatment and preparation method thereof
A technology of photodynamic therapy and nano-medicine, applied in the field of biomedicine, can solve the problem of high dark toxicity of hematoporphyrin, achieve the effects of improving therapeutic effect, improving solubility, and solving dark toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
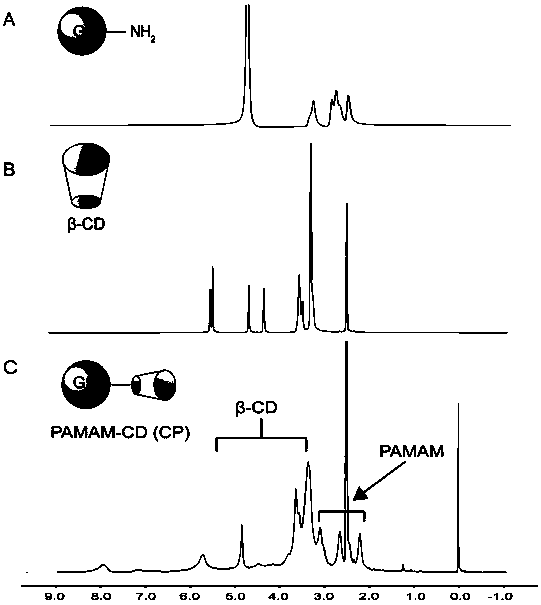
[0041] Weigh β-CD (126.4 mg) and CDI (180.56 mg) and dissolve them in 7 mL of DMSO. After fully stirring the reaction for 6 h, the fifth-generation polyamide-amine dendrimer (G5 PAMAM) (115.9 mg) in DMSO solution (3 mL) was added dropwise to the solution, and stirred at room temperature for 3 days. After the reaction is over, the reaction solution is transferred to a dialysis bag with a molecular weight cut-off of 8000-14000 Da, and dialyzed in secondary water for 3 days. Finally, freeze-dried to obtain a white powdery product (CP), stored at -20°C. Use deuterated DMSO as 1 Test solvent for H-NMR spectrum, from figure 1 It can be seen that cyclodextrin has been successfully coupled to PAMAM.
Embodiment 2
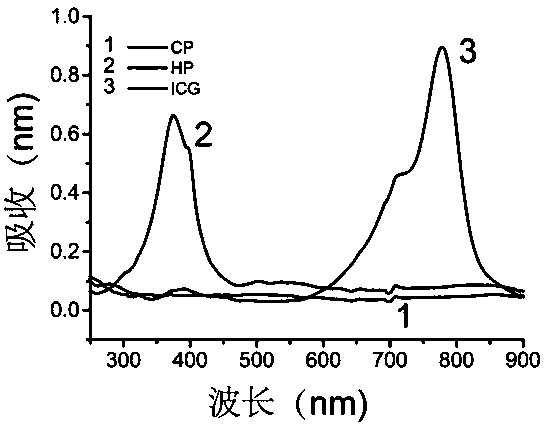
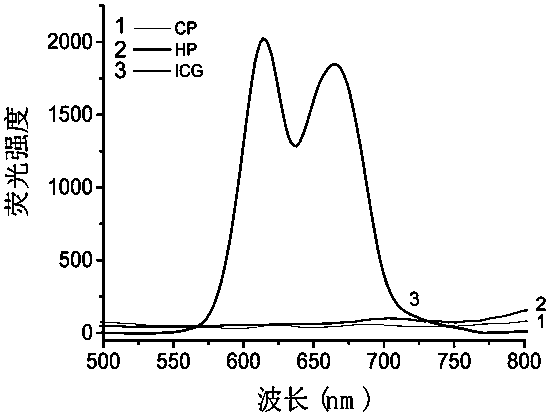
[0043] Take the aqueous solution of CP, ICG, HP to measure the UV absorption, such as figure 2 As shown, HP has characteristic ultraviolet absorption peaks at 374 nm and 500-620 nm, ICG has characteristic ultraviolet absorption peaks at 778 nm, and CP has no ultraviolet absorption peaks at 300-900 nm. Measure the fluorescence intensity of CP, ICG, HP at an excitation wavelength of 401 nm, such as image 3 The HP shown has characteristic peaks of HP at 616nm and 666nm, while CP and CPI have no fluorescence.
Embodiment 3
[0045] Weigh 10mg CP and dissolve it in 8mL DMSO, add ICG (2mg, 1mL DMSO) and HP (0.2mg, 1mL DMSO) dropwise to the CP solution, and stir for 3 days at room temperature and dark. The reaction solution was dialyzed in secondary water for 3 days and freeze-dried to obtain CPHI10. Among them, the encapsulation rate and loading rate of ICG are 15.4%±0.63% and 6.16%±0.03%, respectively, and the encapsulation rate and loading rate of HP are 82.5%±0.25% and 3.30%±0.64%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com