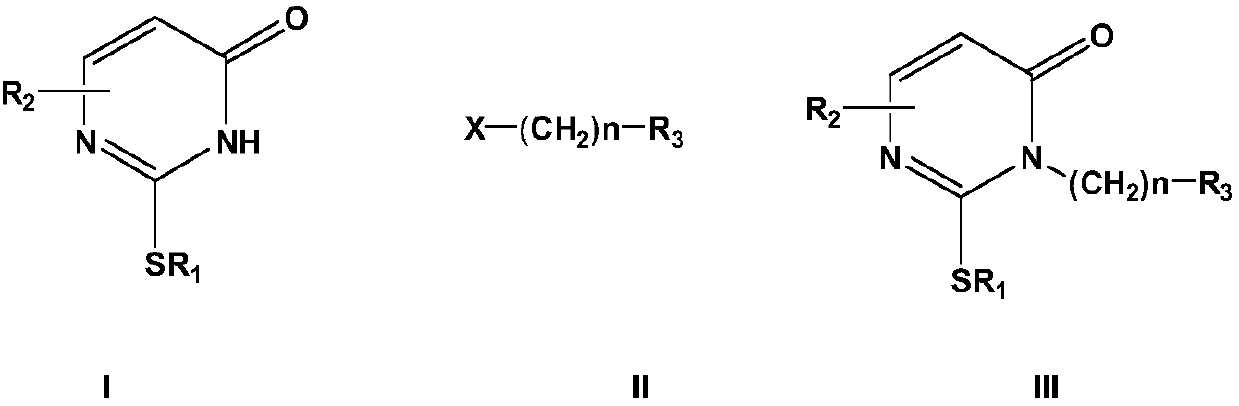
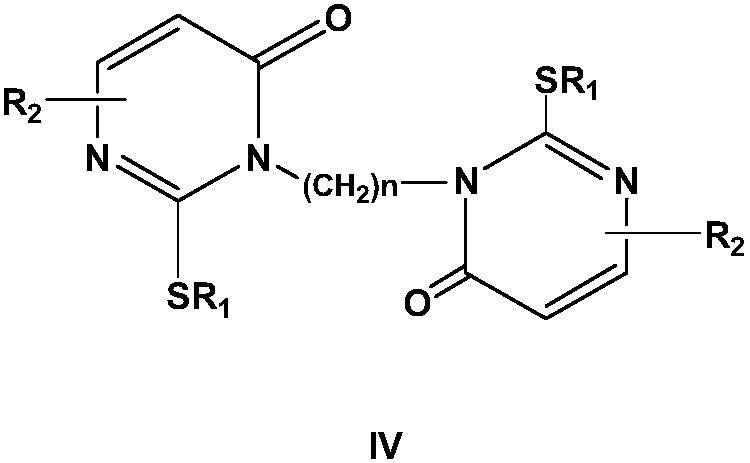
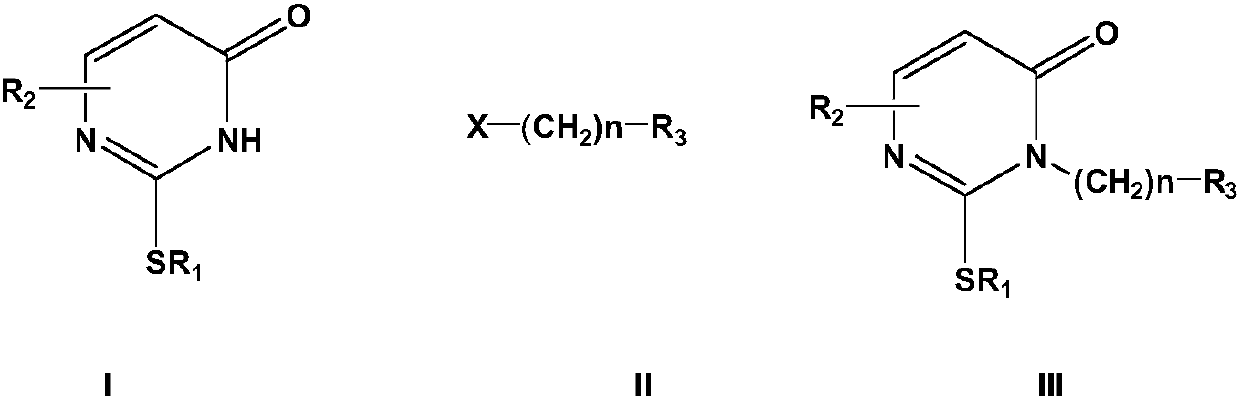
Method for synthesizing nucleoside antiviral drug intermediate thiopyrimidone derivatives
A technology for thiopyrimidinones and a synthesis method, which is applied in the fields of organic synthesis and pharmaceutical intermediate synthesis, can solve the problems of inability to overcome isomer by-products, insufficient purity, increased production cost, etc., and achieves good application prospects and industrialization potential, Resolve the effect of high isomer content and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
[0046] In a nitrogen inert atmosphere, add 10 mmol of the above formula (I) compound, 10 mmol of the alkylating agent Br ( CH2) 4 OAc (4-n-butyl bromoacetate) and 15mmol basic reagent cesium carbonate, heated under reflux under stirring conditions for 1 hour, then added 0.5mmol phase transfer catalyst benzyltriethylammonium chloride and 0.1mmol cocatalyst bromination Tetra-n-butylammonium, under stirring, add 1mmol of dibenzo-15-crown-5 until it is evenly stirred, continue to heat and reflux under stirring for 4 hours, after the reaction is completed, cool the resulting mixture to 0- 15°C, filter, concentrate the filtrate, separate by silica gel column chromatography (the eluent used is a mixed solvent of n-hexane and ethyl acetate with a volume ratio of 5:1), collect the eluate and remove the eluent under reduced pressure , the target compound of the above formula (III) was obtained with a yield of 96.3%, and HPLC analysis showed that the purity was greater than ...
Embodiment 2
[0049]
[0050] In a nitrogen inert atmosphere, add 20 mmol of the above formula (I) compound to 100 mL of the composite solvent (composed of equal volumes of N-methyl-2-pyrrolidone and dioxane) in the reactor, add 10 mmol of the alkylating Reagent Br(CH 2 ) 2 Br and 15mmol basic reagent cesium carbonate, 0.5mmol phase transfer catalyst benzyltriethylammonium chloride and 0.1mmol cocatalyst tetra-n-butylammonium bromide, and then add 1mmol dibenzo-15-crown -5 until the agitation is uniform, and the reaction is heated under reflux for 8 hours under agitation. After the reaction is completed, the resulting mixture is cooled, cooled to 0-15° C., filtered, and the filtrate is concentrated and separated by silica gel column chromatography (the eluent used is volume ratio 5:1 mixed solvent of n-hexane and ethyl acetate), collect the eluent and remove the eluting solvent under reduced pressure, and recrystallize in acetonitrile to obtain the target compound of the above formula (...
Embodiment 3
[0054] The cesium carbonate in Example 1 is replaced by potassium carbonate, and the 1,4-dioxane and DMF composite solvent is replaced by 1,4-dioxane, and other operations are unchanged, and the compound of the above formula (III) is obtained. The yield was 85.1%, and HPLC analysis showed that the purity was greater than 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com