Device for producing alkylated aromatic compounds
A technology for alkylating aromatic compounds, applied in the direction of hydrocarbons, hydrocarbons, carbon compound catalysts, etc., can solve the problems of limited capacity and low adsorption efficiency of adsorbents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
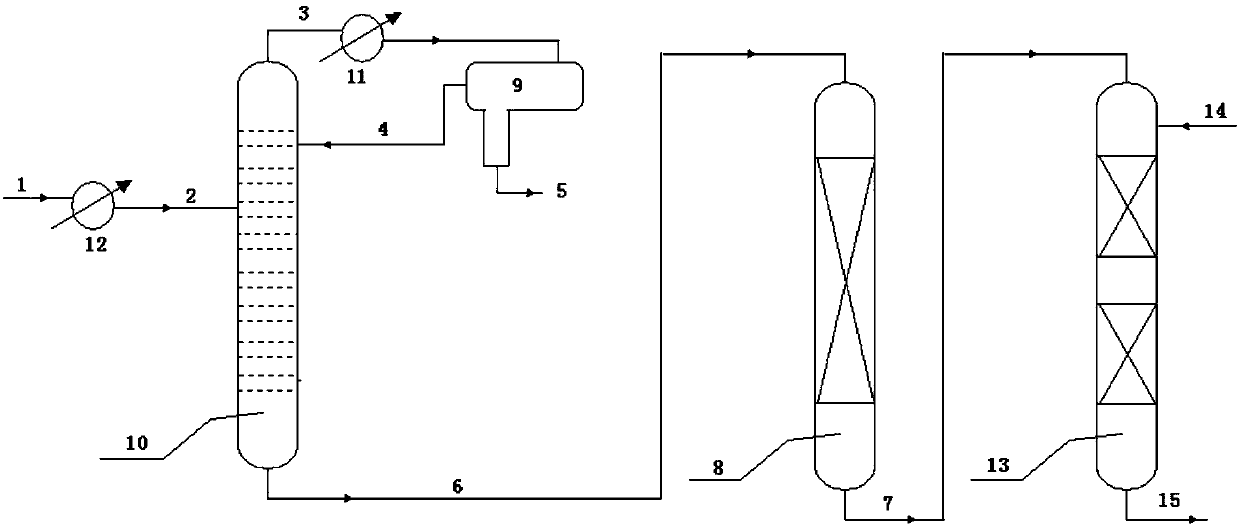
[0043] press figure 1 In the device, the raw material benzene has a water content of 200ppm, a total nitrogen content of 3ppm, a total sulfur content of 0.7ppm, a distillation tower operating pressure of 0.01Pa, a tower top temperature of 80°C, and a tower bottom temperature of 82°C. The adsorbent bed is filled with 100g acid clay, the adsorbent bed temperature is 82℃, the pressure is 0.5MPa, and the benzene weight space velocity is 5.0h -1 . After continuous operation for 100 hours, the total nitrogen content at the outlet of the adsorbent bed was analyzed by a sulfur and nitrogen analyzer to be 0.05 ppm, and the total sulfur content was 0.06 ppm. .
[0044] Using the purified benzene as the raw material, the alkylation of benzene and propylene is the target reaction. The reaction conditions are: fixed bed reactor, catalyst is acid Beta zeolite, reaction temperature 160℃, reaction pressure 3.0MPa, propylene weight space velocity 10.0h -1 , The molar ratio of benzene to propyle...
Embodiment 2
[0046] [Example 1] Purified benzene is used as the raw material, and the alkylation of benzene and ethylene is the target reaction. The reaction conditions are: fixed bed reactor, the catalyst is acid Beta zeolite, the reaction temperature is 205°C, and the reaction pressure is 4.5MPa. Ethylene weight space velocity 3.0h -1 , The molar ratio of benzene to ethylene is 4.0, the continuous reaction is 200 hours, and the ethylene conversion rate reaches 99%.
Embodiment 3
[0048] press figure 1 In the device, the raw material benzene has a water content of 500ppm, a total nitrogen content of 3ppm, a total sulfur content of 1.6ppm, a distillation tower operating pressure of 0.6MPa, a tower top temperature of about 140°C, and a tower bottom temperature of 160°C. The adsorbent bed is filled with 100g acid Beta zeolite, the adsorbent bed temperature is 160℃, the pressure is 2.0MPa, and the benzene weight space velocity is 3.0h -1 . After continuous operation for 100 hours, the total nitrogen content at the outlet of the adsorbent bed was analyzed by a sulfur-nitrogen analyzer to be 0.05 ppm, and the total sulfur content was 0.07 ppm.
[0049] Using the purified benzene as the raw material, the alkylation of benzene and propylene is the target reaction. The reaction conditions are: fixed bed reactor, catalyst is acid Beta zeolite, reaction temperature 160℃, reaction pressure 3.0MPa, propylene weight space velocity 10.0h -1 , The molar ratio of benzene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com