Pichia pastoris expressing recombinant Thanatin antibacterial peptide
A Pichia pastoris and antibacterial peptide technology, applied in the fields of biotechnology and genetic engineering, can solve the problems of low expression of death factor and high production cost, and achieve the effects of reducing production cost and facilitating separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Construction of Deathin Expression Strain
[0059] 1.1 Design of the deadin THA gene based on the codon preference of Pichia pastoris
[0060] According to Pichia pastoris ( Pichia pastoris ) codon preference:
[0061] Amino Acid Codon Frequency ‰ Quantity
[0062] Phe UUU 24.1 1963
[0063] Phe UUC 20.6 1675
[0064] Leu UUA 15.6 1265
[0065] Leu UUG 31.5 2562
[0066] Ser UCU 24.4 1983
[0067] Ser UCC 16.5 1344
[0068] Ser UCA 15.2 1234
[0069] Ser UCG 7.4 598
[0070] Tyr UAU 16.0 1300
[0071] Tyr UAC 18.1 1473
[0072] STOP UAA 0.8 69
[0073] STOP UAG 0.5 40
[0074] Cys UGU 7.7 626
[0075] Cys UGC 4.4 356
[0076] STOP UGA 0.3 27
[0077] Trp UGG 10.3 834
[0078] Leu CUU 15.9 1289
[0079] Leu CUC 7.6 620
[0080] Leu CUA 10.7 873
[0081] Leu CUG 14.9 1215
[0082] Pro CCU 15.8 1282
[0083] Pro CCC 6.8 553
[0084] Pro CCA 18.9 1540
[0085] Pro CCG 3.9 320
[0086] His CAU 11.8 960
[0087] His CAC 9.1 737
[0088] H...
Embodiment 2
[0138] Example 2 Induced Expression of Recombinant Deathin
[0139] Pick the Pichia pastoris cells expressing deathin prepared in Example 1, inoculate them in 25 mL of BMGY medium, and culture them at 30° C. and 220 rpm for 24 hours to prepare a primary seed solution.
[0140] Inoculate 20 mL of primary seed liquid into 200 mL of BMGY medium to prepare secondary seed liquid, and culture at 30°C and 220 rpm for 24 hours.
[0141] All the secondary seed liquids were inoculated in a 5L fermenter (2L BSM medium), the temperature was controlled at 30°C±0.5°C, the dissolved oxygen was 20%±5%, and the pH=5.0±0.5.
[0142] After the basal glycerol is exhausted, add 10% glycerol. After the glycerol is exhausted, until the dissolved oxygen suddenly rises, starve for half an hour, add methanol to induce the expression of deadin, and induce the expression of death factor for a total of 120 hours. Centrifuge (6000×g, 5min) to take the fermentation supernatant to test.
Embodiment 3
[0143] Example 3 Detection of Recombinant Deathin Protein Concentration and Protein Electrophoresis
[0144] The total protein concentration of the supernatant of the fermentation broth in the example was measured by the Bradford method, and the results showed that the total protein concentration of the supernatant of the fermentation supernatant induced by the deadin expression strain for 120 hours was 0.67 g / L.
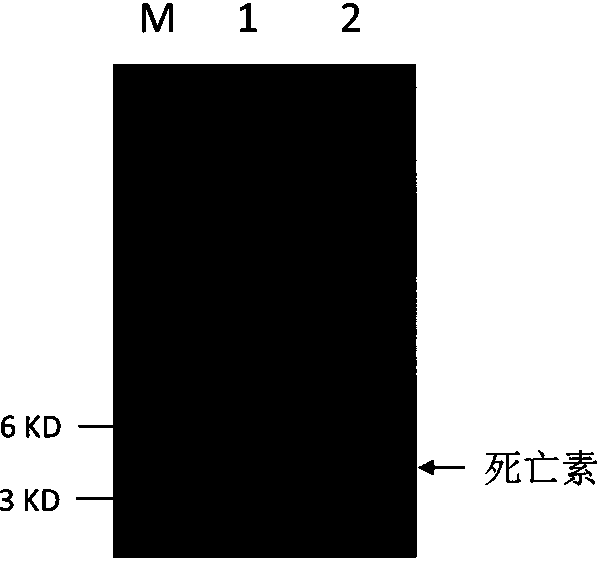
[0145] see image 3 , Tricine-SDS-PAGE protein electrophoresis analysis of the content of deathin in the fermentation supernatant showed that after protein electrophoresis in the fermentation supernatant, there was a protein band with a target size between 3 and 6KD, and the molecular weight of the protein band was similar to that of deathin. The molecular weight is similar, about 2.4KD; through software analysis, the content of death protein is more than 90%. The total protein concentration of the fermentation supernatant was 0.67g / L, and the corresponding death p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com