Medicine composition for washing nasal cavity and preparation method of medicine composition
A composition and drug technology, applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., to achieve the effects of stable curative effect, accelerated recovery of health, and clear effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0049] The following describes but does not limit the present invention through the description of specific embodiments of the present invention.
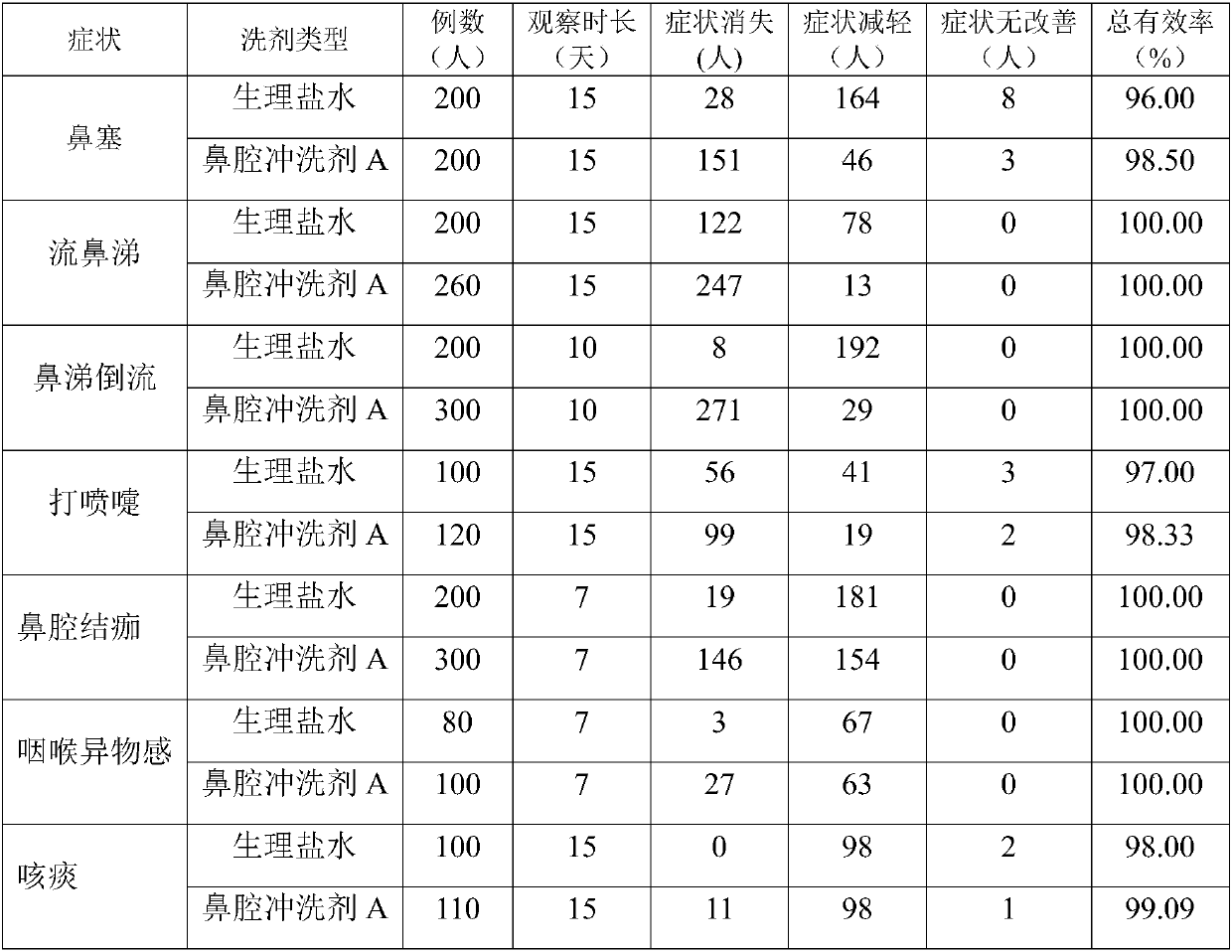
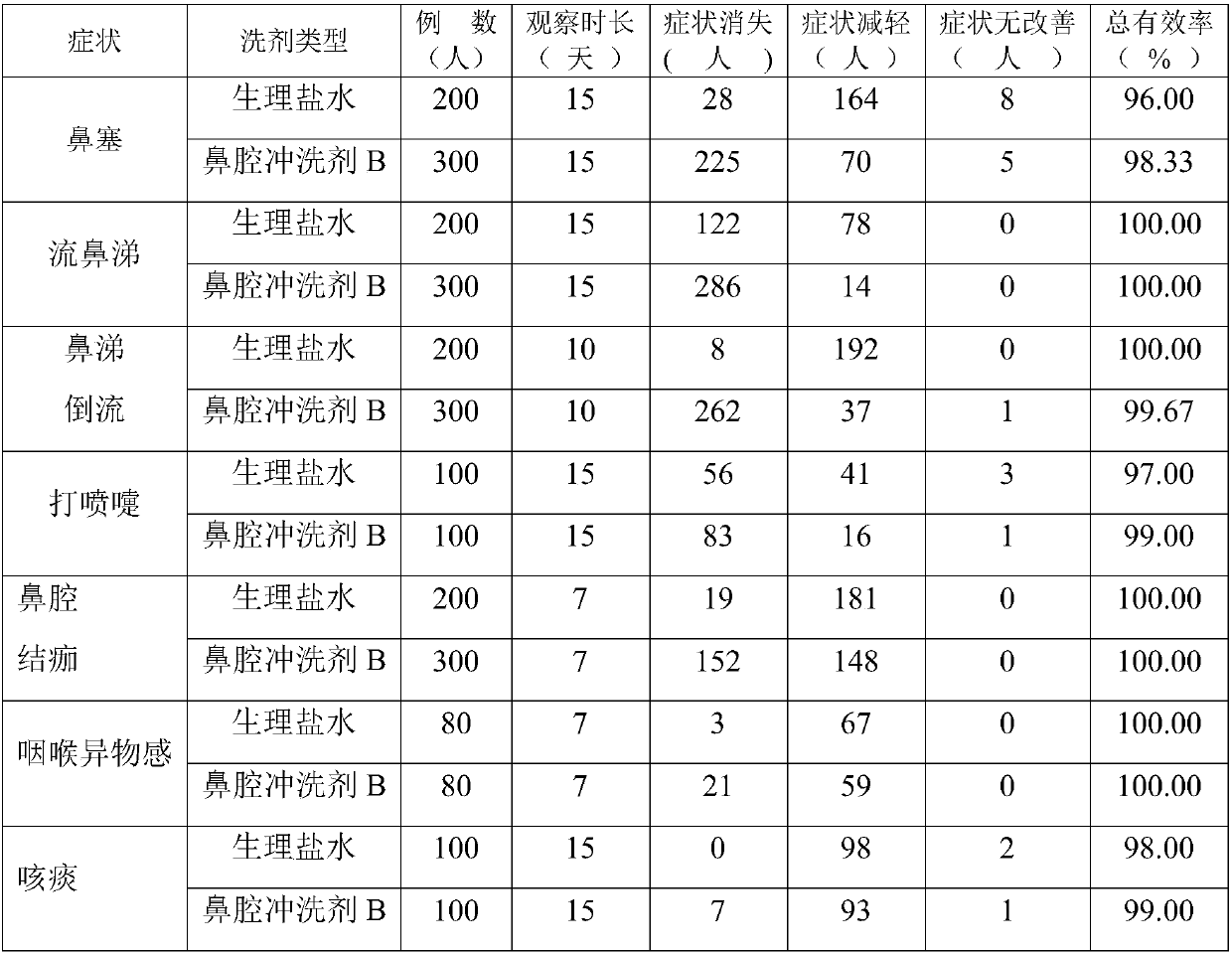
[0050] During two years of clinical application, the inventor adjusted the dosage of the crude drug within the following weight ratio range, all of which could achieve the treatment of nasal congestion, runny nose, bloody nose, backflow of nasal mucus, sneezing, scab in the nasal cavity, dry and itchy nasal cavity, throat Various nasal cavity and upper respiratory tract infections such as foreign body sensation, excessive phlegm, expectoration, bloody sputum, throat inflammation, etc.: the weight ratio of raw materials is 35-75 parts of sodium chloride, 1-4 parts of lysozyme or sodium chloride 35-85 parts, 0.1-0.5 parts of glycine, 1-4 parts of lysozyme.
[0051] For the convenience of application, on the basis of raw materials, pharmaceutically acceptable excipients can be added to make common powders, lotions, concentrates, spray...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com