Single-stranded TCR-T carrier and single-stranded TCR-T cell production process
A TCR-T, single-chain technology, applied in receptors/cell surface antigens/cell surface determinants, genetically modified cells, and the introduction of foreign genetic material using vectors, which can solve the problem of long vector sequences and affect the expression of TCR-T. and quality monitoring, safety risks and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0050] step 1)
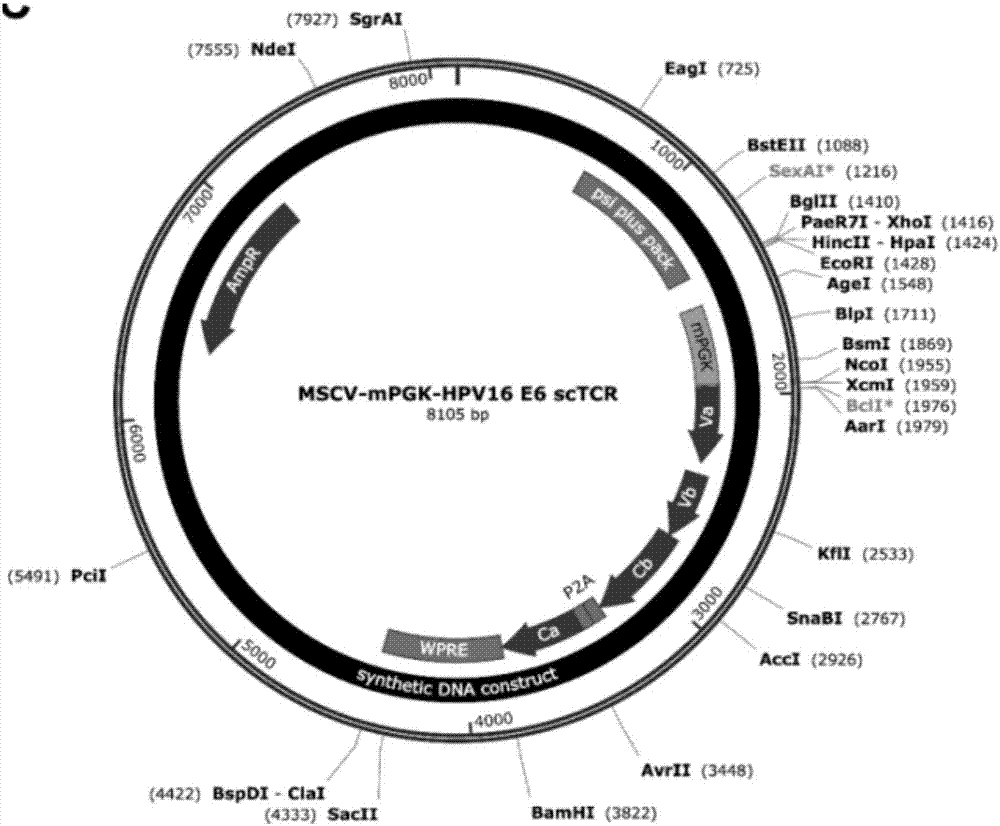
[0051] Design and construction of HPV-16E6 single-chain TCR-T vector
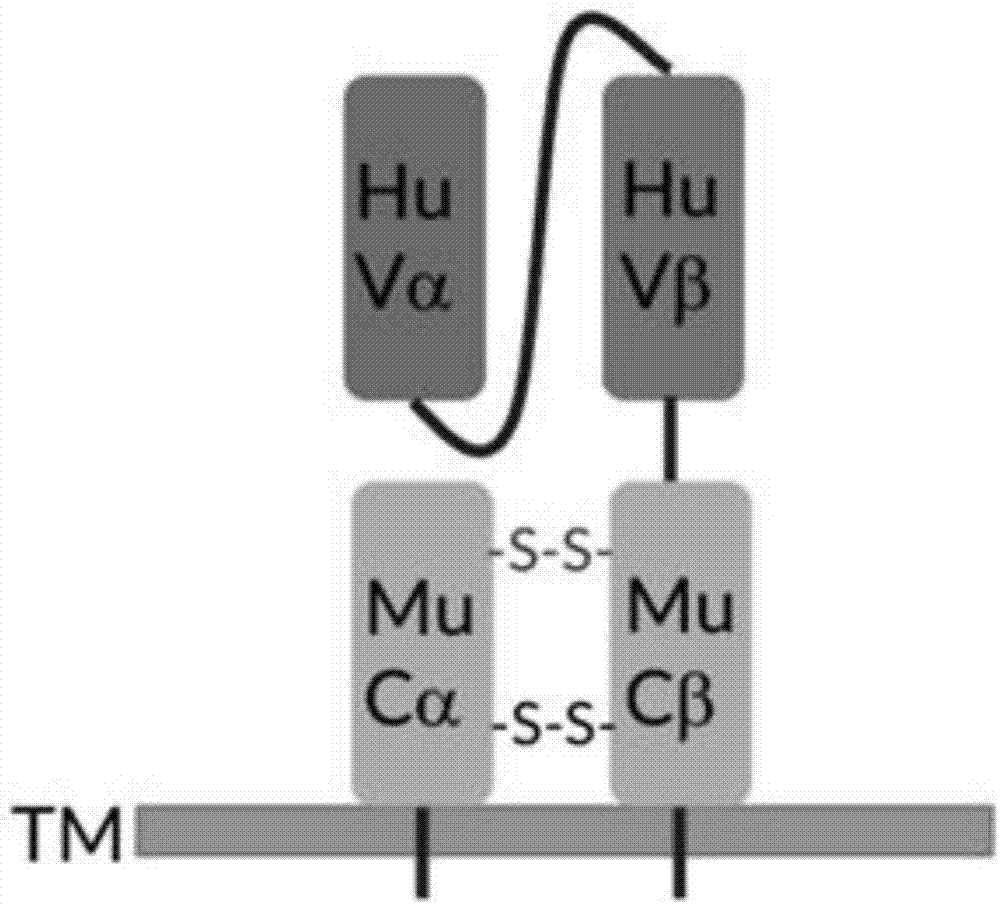
[0052] The general structure of HPV-16E6 single-chain TCR-T is as follows Figure 1A As shown, the antigen recognition parts Hu Vα and Hu Vβ are composed of four repeated GGGS-linked polypeptides. The single-chain part is connected with the murine TCR Cβ chain, including the transmembrane region and the newly generated disulfide bond site after mutation (red-S-S-marked). In addition, the single-chain TCR contains the mouse TCR Cα chain, including the transmembrane region and the disulphide bond site generated after mutation, and its position can be paired with the mouse Cβ chain, so that the mouse Cα chain and the mouse Cβ chain have two a disulfide bond pairing site. In terms of vector design, in order to keep the expression levels of Hu Vα-Linker-Hu Vβ-Mu Cβ peptide chain and Mu Cα peptide chain equivalent, the two peptide chains were linked by P2A sequence. The P2A sequence can be sel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com