High-selectivity magnetic dye adsorbent and preparation method thereof
A high-selectivity, adsorbent technology, applied in chemical instruments and methods, adsorption water/sewage treatment, water pollutants, etc., can solve problems such as limited adsorption capacity, weak selectivity, and cumbersome recovery process of adsorbents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
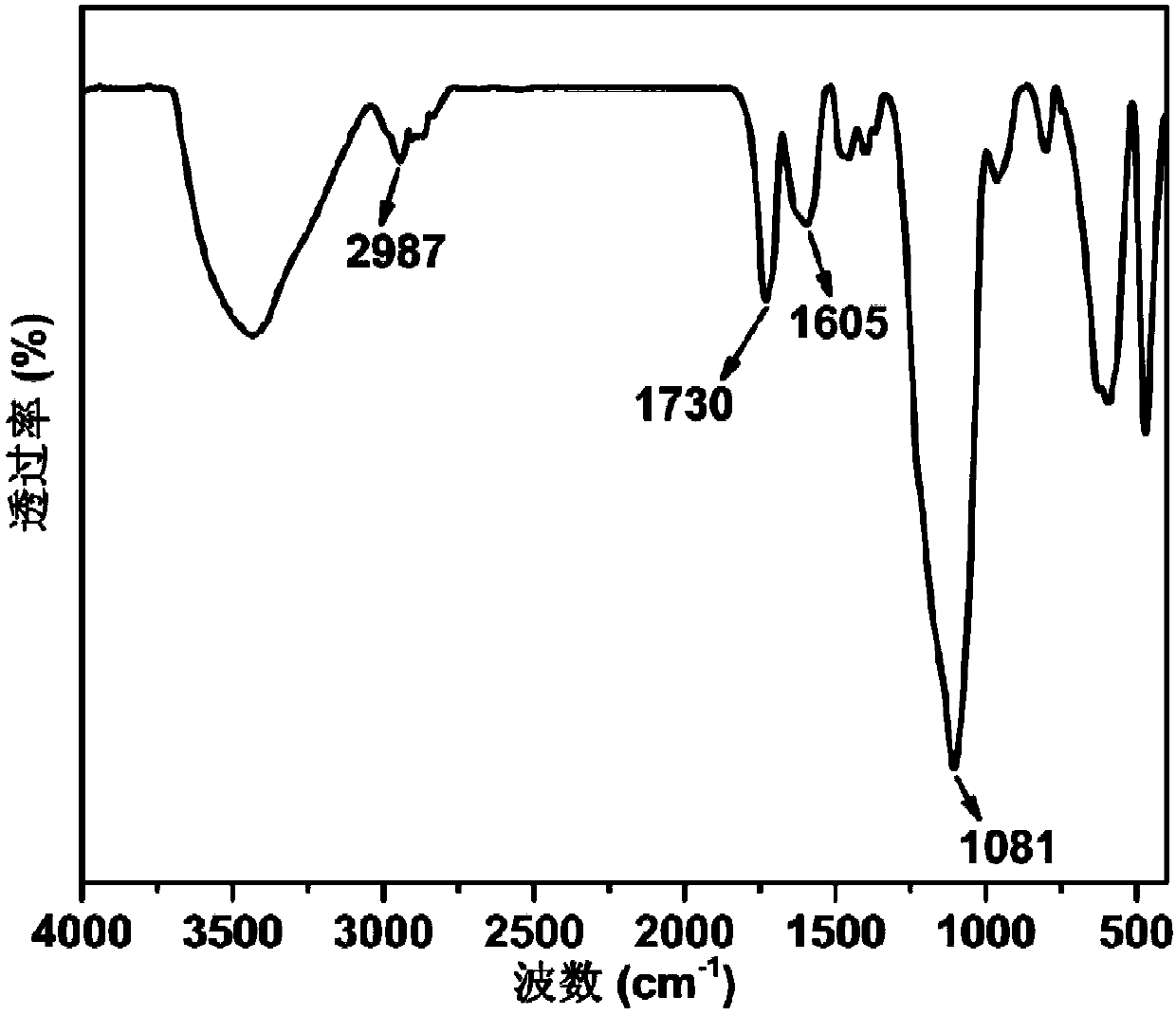
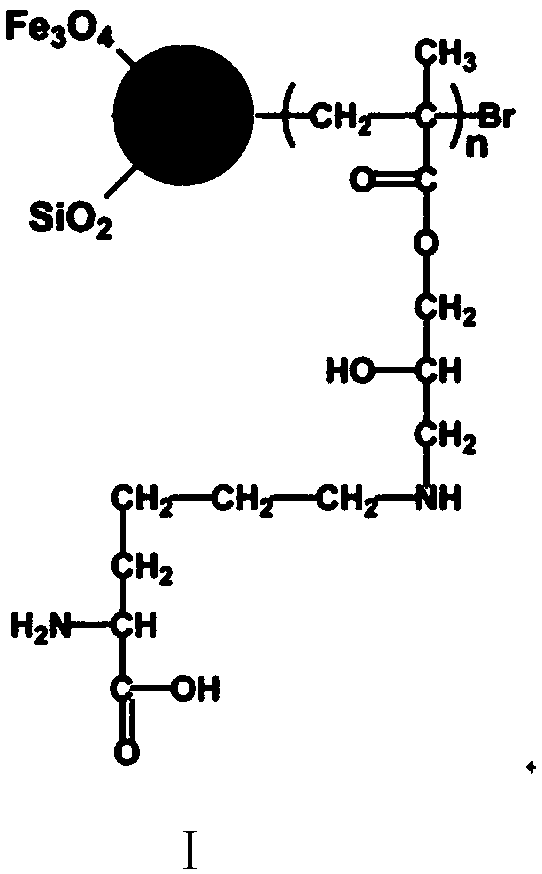
[0061] A preparation method of a highly selective magnetic dye adsorbent, the steps are as follows:
[0062] (1) Magnetic Nano Fe 3 o 4 preparation of
[0063] 2.7g FeCl 3 ·6H 2 O and 7.2g of anhydrous NaAc were uniformly dispersed in 100mL of ethylene glycol by ultrasonic, stirred overnight at room temperature, heated to 200°C in an autoclave for 18 hours, and the solid obtained by magnetic separation was dispersed and washed 5 times in absolute ethanol , and then vacuum-dried at room temperature to obtain magnetic nano-Fe 3 o 4 ;
[0064] (2) Nano Fe 3 o 4 -SiO 2 preparation of
[0065] The magnetic nano Fe that 50mg step (1) obtains 3 o 4 Ultrasonic dispersion in 150mL of absolute ethanol, adding 5mL of ammonia water with a mass concentration of 26% and 3mL of tetraethyl orthosilicate, raising the temperature to 50°C and stirring mechanically for 24h, applying a magnetic field to separate, and washing with absolute ethanol and deionized water alternately for 5 ...
Embodiment 2
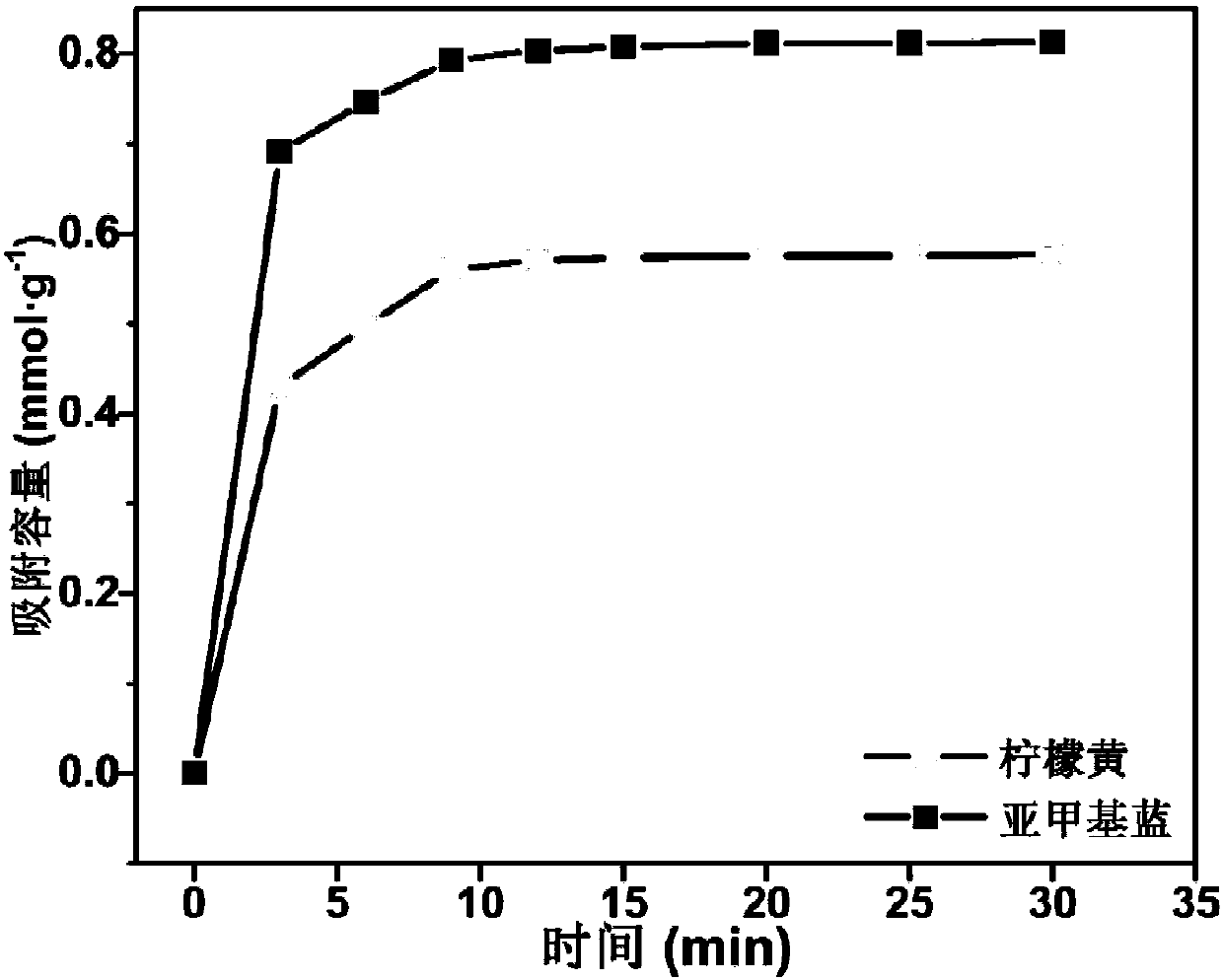
[0081] A preparation method of a highly selective magnetic dye adsorbent, as described in Example 1, the difference is that the amount of tetraethyl orthosilicate used in step (2) is 1mL, and the grafting ratio of the prepared adsorbent is 51.60 %.
[0082] According to the method of Example 1, the adsorption capacity of the adsorbent to the organic dye was tested. The adsorbent prepared in the present embodiment is in the environment of pH=4 to tartrazine (mass concentration is 1000mg L -1 ) has an adsorption capacity of 0.357mmol·g -1 ; In the environment of pH=10, to methylene blue (mass concentration is 1000mg L -1 ) has an adsorption capacity of 0.511mmol·g -1 .
Embodiment 3
[0084] A preparation method of a highly selective magnetic dye adsorbent, as described in Example 1, the difference is that the amount of tetraethyl orthosilicate used in step (2) is 5mL, and the grafting ratio of the prepared adsorbent is 88.60 %.
[0085] According to the method of Example 1, the adsorption capacity of the adsorbent to the organic dye was tested. The adsorbent prepared in the present embodiment is in the environment of pH=4 to tartrazine (mass concentration is 1000mg L -1 ) has an adsorption capacity of 0.601mmol·g -1 ; In the environment of pH=10, to methylene blue (mass concentration is 1000mg L -1 ) has an adsorption capacity of 0.826mmol·g -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com