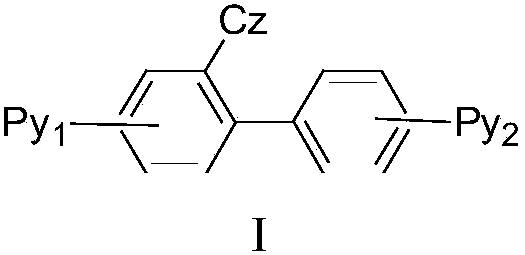
Carbazole pyridine derivative, purpose of carbazole pyridine derivative and organic electroluminescence device
A technology of carbazole pyridine and derivatives, which is applied in the field of organic electroluminescent materials, can solve the problems of unbalanced charge in the light-emitting layer, reduced device efficiency, difficult electron flow, etc., and achieves high luminescence purity, good thermal stability, and high luminescence. The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
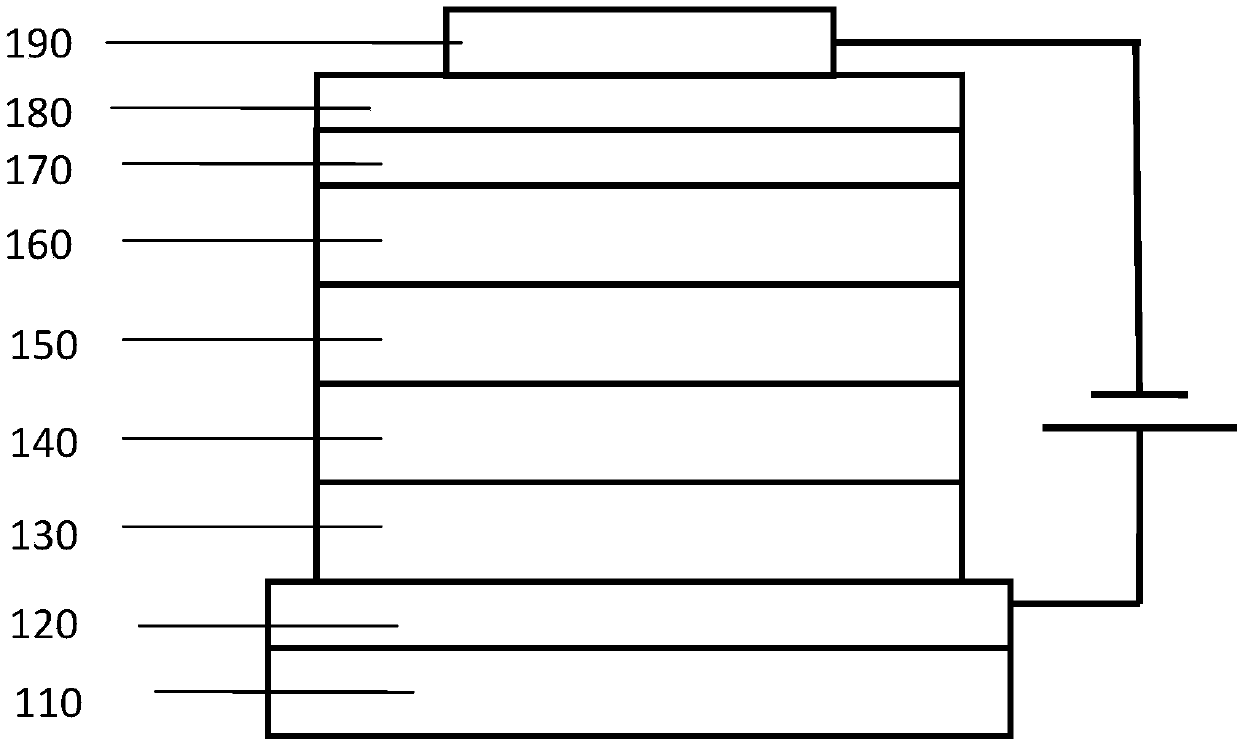
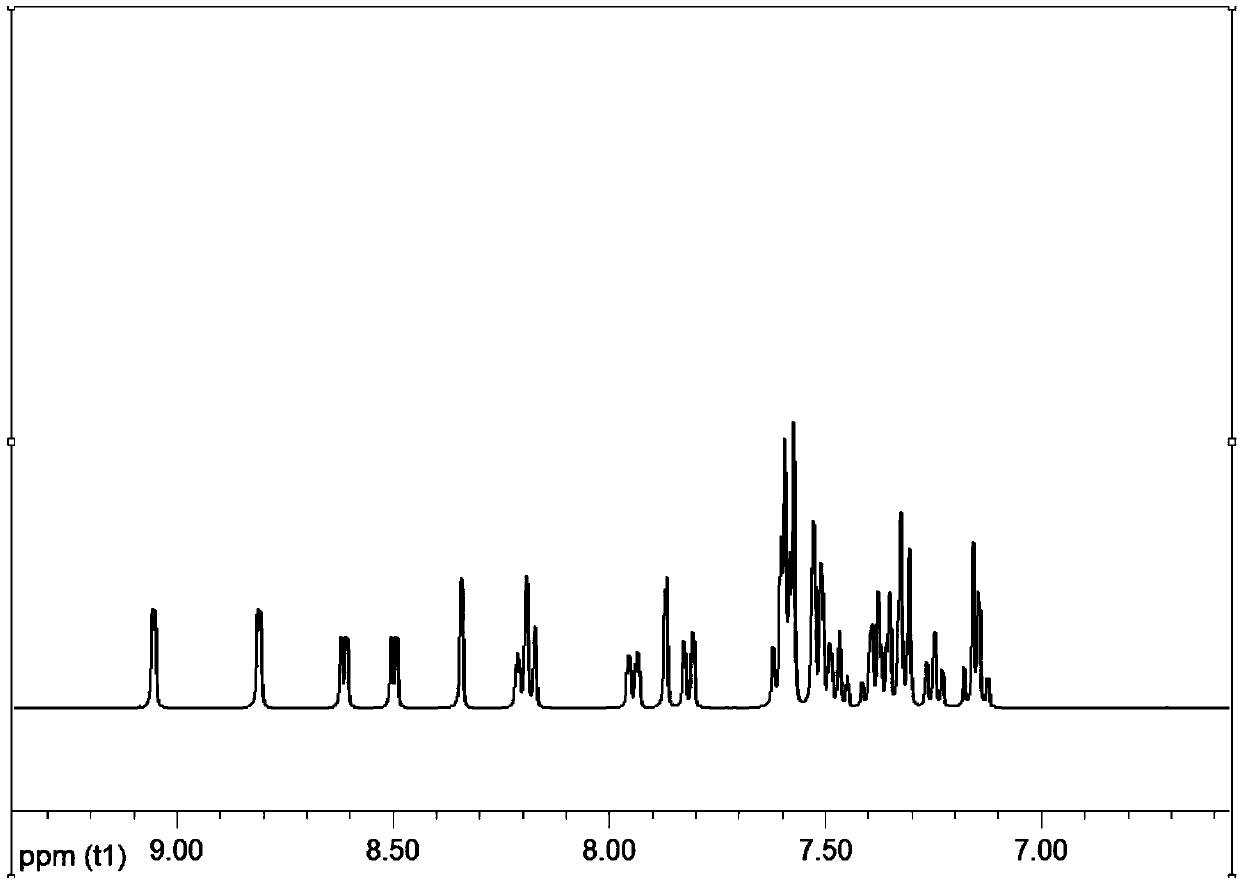
Image
Examples
Embodiment 1
[0044] Synthetic route of compound 1
[0045]
[0046] The synthetic method of intermediate 1
[0047] In the flask, add 2-iodo-4,4'-dibromobiphenyl (2.5g, 5.8mmol), 9-phenylcarbazole-3-boronic acid (1.7g, 5.8mmol), potassium carbonate (1.6g, 11.6mmol), tetrahydrofuran (20mL) and water (10mL), tetrakistriphenylphosphine palladium (0.2g), heated to reflux under nitrogen protection for 10 hours, cooled, extracted with dichloromethane, dried, concentrated, and the crude product was passed through the column Chromatographic purification afforded 1.5 g of the product in a 46% yield.
[0048] The synthetic method of compound 1
[0049] In a flask, add intermediate 1-1 (1.5 g, 2.7 mmol), pinacol 3-pyridineboronate (1.7 g, 8 mmol), potassium carbonate (0.74 g, 5.4 mmol), tetrahydrofuran (20 mL) and water ( 10mL), tetrakistriphenylphosphine palladium (0.2g), heated to reflux under nitrogen protection for 24 hours, cooled, extracted with dichloromethane, dried, concentrated, and t...
Embodiment 2
[0051] Synthetic route of compound 6
[0052]
[0053] The synthetic method of intermediate 6-1
[0054] In the flask, add 2-bromo-7,7-dimethyl-5-phenyl-indeno[2,1-B]carbazole (5g, 11.4mmol), dry tetrahydrofuran (50mL), and cool under nitrogen To -78 degrees, slowly add 2.5M n-butyllithium (5mL, 12.5mmol) dissolved in n-hexane, keep the reaction for 2 hours, then slowly add triisopropyl borate (2.4g, 12.5mmol), continue the reaction for 1 hour , slowly rise to room temperature and react for 12 hours, slowly add 2N dilute hydrochloric acid aqueous solution, adjust the pH value to be less than 7, add dichloromethane for extraction, dry, concentrate, add n-hexane, stir, and filter to obtain 3.8g of the product, the yield is 83%.
[0055] The synthetic method of intermediate 6-2
[0056] The synthesis method is the same as that of intermediate 1-1, the raw material used is intermediate 6-1, and the yield is 51%.
[0057] The synthetic method of compound 6
[0058] The synthe...
Embodiment 3
[0060] Synthetic route of compound 16
[0061]
[0062] The synthetic method of intermediate 16-1
[0063] The synthesis method is the same as that of intermediate 1-1, the raw material used is 4-carbazolylphenylboronic acid, and the yield is 62%.
[0064] The synthetic method of compound 16
[0065] The synthesis method is the same as that of compound 1, the raw material used is intermediate 16-1, and the yield is 68%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com