Manidipine hydrochloride tablet for treating hypertension and preparation method thereof
A technology of manidipine hydrochloride and manidipine hydrochloride, which is applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, and pill delivery, etc., can solve the problems of low bioavailability and low dissolution rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
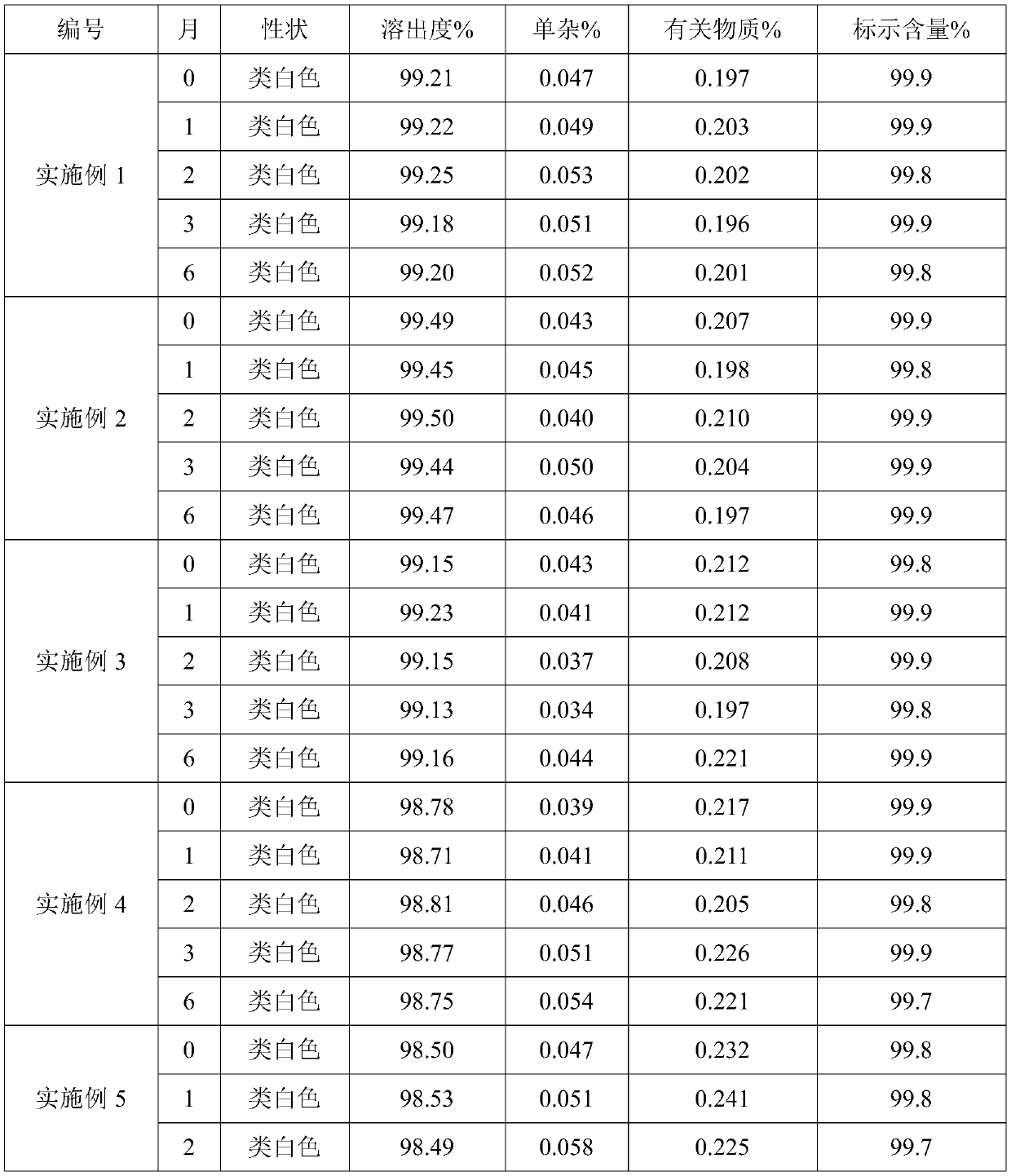
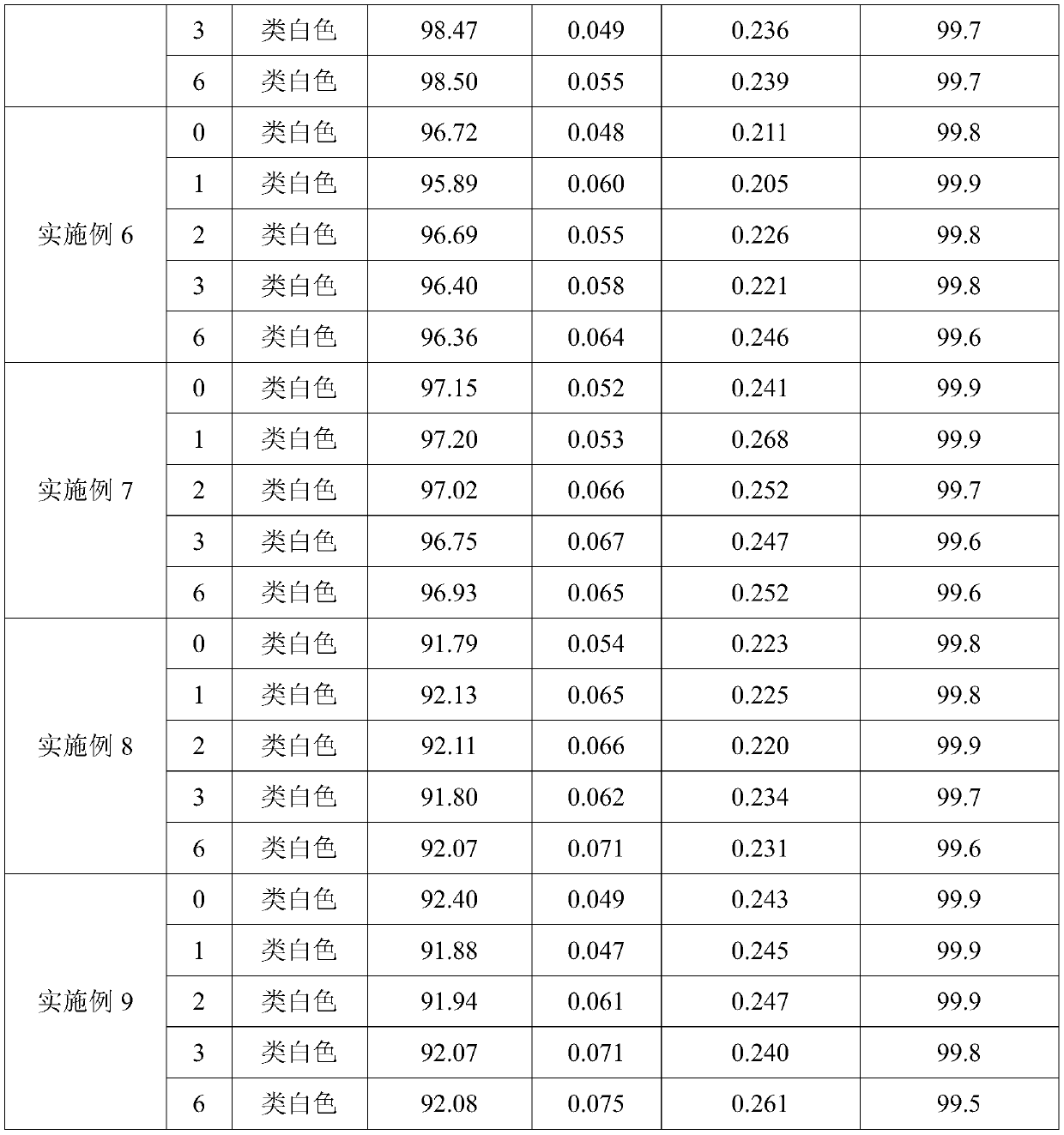
Examples
Embodiment 1
[0023] The preparation of manidipine hydrochloride tablet
[0024] (1) 25g polyethylene glycol 6000 is melted;
[0025] (2) 10 g of manidipine hydrochloride, 0.5 g of sodium lauryl sulfonate and 60 g of lactose are pulverized to an average particle size of 10 μm, and then mixed with molten polyethylene glycol 6000 by an electrostatic powder spray gun;
[0026] (3) After raising the temperature of the material mixed in step (2) to 125°C, stir at a speed of 250r / min for 1-2min, cool down to 75°C, add 0.9g of magnesium stearate and stir for 30 minutes at a speed of 80r / min , then transfer the material to a drying oven, dry at 80° C. for 6 to 8 hours, and compress the dried material into tablets to obtain 1000 manidipine hydrochloride tablets.
Embodiment 2
[0028] The preparation of manidipine hydrochloride tablet
[0029] (1) Polyethylene glycol 6000 of 37.5g is melted;
[0030] (2) 10 g of manidipine hydrochloride, 3.1 g of sodium lauryl sulfonate and 62.5 g of lactose are pulverized to an average particle size of 30 μm, and then mixed with molten polyethylene glycol 6000 by an electrostatic powder spray gun;
[0031] (3) After raising the temperature of the mixed material in step (2) to 120°C, stir at 240r / min for 1-2min, cool down to 75°C, add 0.6g of magnesium stearate and stir for 30 minutes at 100r / min , then transfer the material to a drying oven, dry at 80° C. for 6 to 8 hours, and compress the dried material into tablets to obtain 1000 manidipine hydrochloride tablets.
Embodiment 3
[0033] The preparation of manidipine hydrochloride tablet
[0034] (1) 30g polyethylene glycol 6000 is melted;
[0035] (2) 10 g of manidipine hydrochloride, 0.2 g of sodium lauryl sulfonate and 80 g of lactose are pulverized to an average particle size of 20 μm, and then mixed with molten polyethylene glycol 6000 by an electrostatic powder spray gun;
[0036] (3) After raising the temperature of the mixed material in step (2) to 130°C, stir at a speed of 280r / min for 1-2min, cool down to 85°C, add 1g of magnesium stearate and stir for 30 minutes at a speed of 80r / min, The material is then transferred to a drying oven, dried at 60° C. for 6 to 8 hours, and the dried material is compressed into tablets to obtain 1000 manidipine hydrochloride tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com