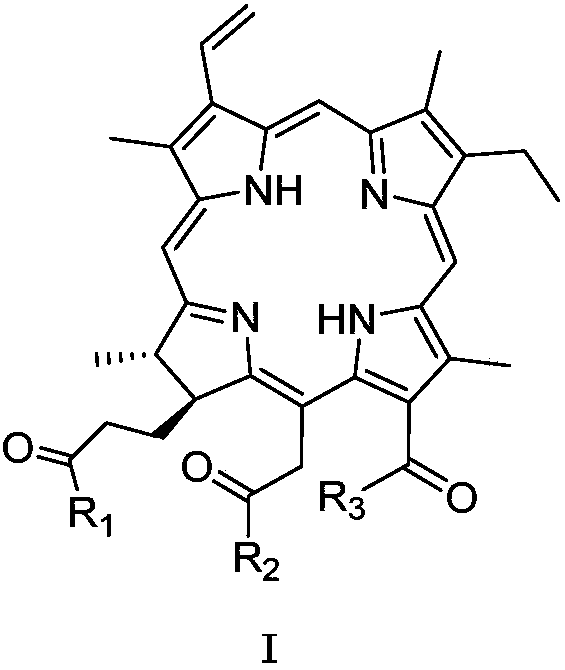
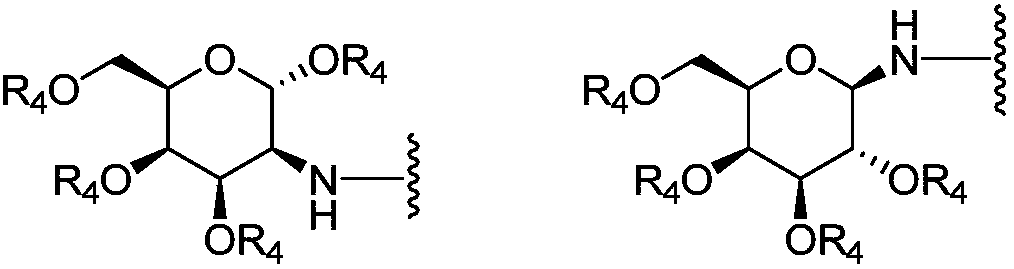
Chlorin galactoside compounds as well as preparation method and application thereof
A technology of galactoside and chlorin, which is applied in the field of chemical medicine, can solve the problems of small absorption coefficient, complex method, poor tumor cell selectivity, etc., and achieves the effects of easy control of conditions, simple synthesis method and favorable production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthesis of compound 3b
[0049] Compound 1 (CHC) (100.0 mg) was dissolved in DMF (1.7 ml). N 2 Protected and stirred in an ice bath. EDCI (38.5 mg) was added, stirring continued in the ice bath, and the ice bath was removed. After stirring at room temperature for 2.5h, 1,3,4,6-tetra-O-acetyl-2-amino-2-deoxy-beta-D-galactopyranose hydrochloride (83.3 mg) was dissolved in DMF ( 1ml), add 40μl of triethylamine and mix thoroughly. Add it into the reaction solution, stir at room temperature, and monitor the reaction termination by TLC. The reaction solution was diluted with 50 ml of dichloromethane, and extracted with aqueous citric acid (25 mL×3). The organic phase was dried, filtered and the filtrate was concentrated. Separation by column chromatography, the elution condition was (dichloromethane / methanol=9:1), and the intermediate product 2b (57.8mg) was obtained; the compound 2b was dissolved in DMF (0.6ml), added iodomethane (31 μl), carbonic acid Potassium (15...
Embodiment 2
[0053] Synthesis of compound 6b
[0054] Dissolve methylated chlorophyllin a (100.0 mg) in CHCl 3 (1.8ml), N 2 Protected and stirred in an ice bath. EDCI (38.5 mg) was added, stirring continued in the ice bath, and the ice bath was removed. After stirring at room temperature for 2.5h, 1,3,4,6-tetra-O-acetyl-2-amino-2-deoxy-beta-D-galactopyranose hydrochloride (83.1 mg) was dissolved in DMF ( 1ml), add 40μl of triethylamine and mix thoroughly. Add it into the reaction solution, stir at room temperature, and monitor the reaction termination by TLC. The reaction solution was diluted with 50 ml of dichloromethane, and extracted with aqueous citric acid (25 mL×3). The organic phase was dried, filtered and the filtrate was concentrated. Separation by column chromatography, the elution condition was (dichloromethane / methanol=9:1), and the intermediate product 5b (55.1mg) was obtained; the compound 5b was dissolved in methanol (0.6ml), and 1 times the amount (molar ratio) of met...
Embodiment 3
[0058] Synthesis of Compound 11
[0059]
[0060] Compound 1 (100 mg) was dissolved in 5% H 2 SO4 / MeOH solution (2ml), N 2 Protection, stirring at room temperature, TLC monitoring reaction termination. Rotary evaporator concentrated to remove methanol. The reaction solution was diluted with dichloromethane (25ml), washed with deionized water, aqueous sodium bicarbonate solution, and saturated NaCl solution once (25ml). The organic phase was dried, filtered and the filtrate was concentrated. After separation by column chromatography, the elution condition was (dichloromethane / methanol=19:1), and 96.2 mg was obtained with a yield of 97%. Compound 8: 1 H NMR (400MHz, CDCl3) δ9.64(1H, s, 10-H), 9.50(1H, s, 5-H), 8.72(1H, s, 20-H), 8.01(1H, dd, J= 17.6, 11.6Hz, 3 1 -H),6.32(1H,dd,J=17.6Hz,1.2Hz,3 2a -H),6.12(1H,dd,J=11.6,1.2Hz,3 2b -H),5.51(1H,d,J=18Hz,15 1a -H),5.26(1H,d,J=18Hz,15 1b -H),4.46(1H,q,J=7.6Hz,18-H),4.12(1H,d,J=7.2Hz,17-H),3.82(3H,s,12 1 -H),3.74(2H,dd,J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com