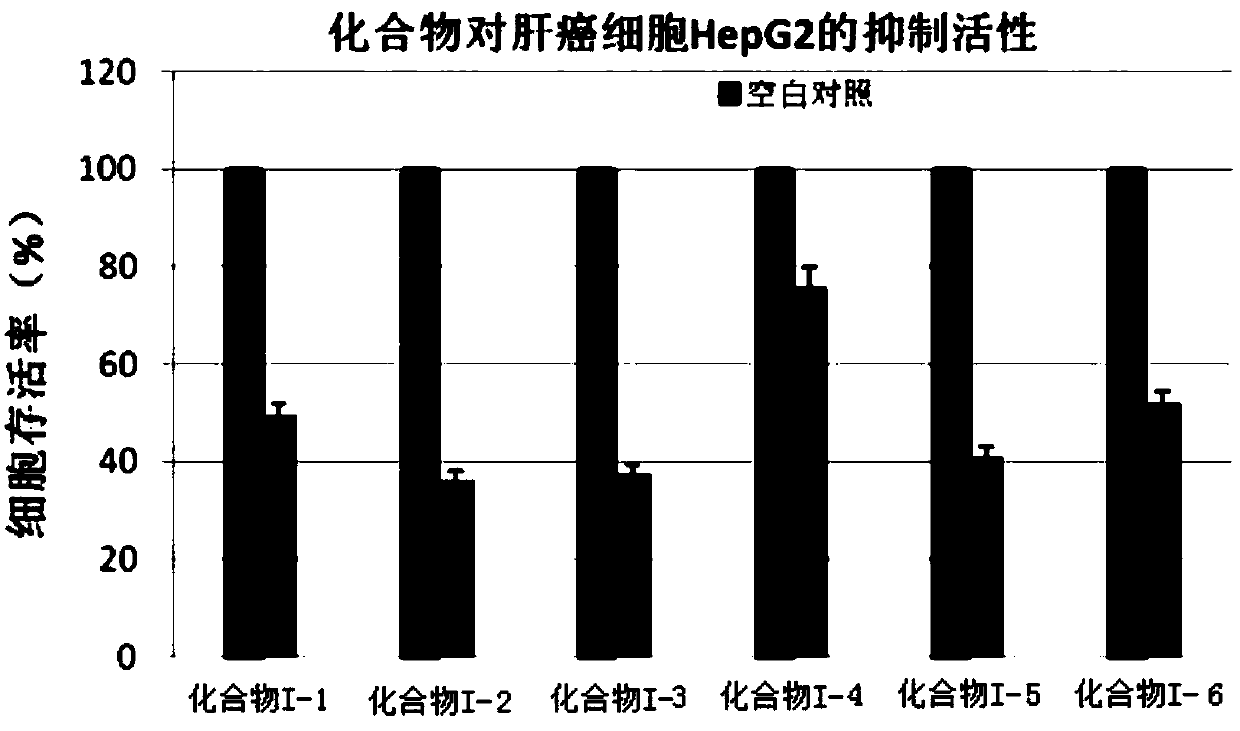
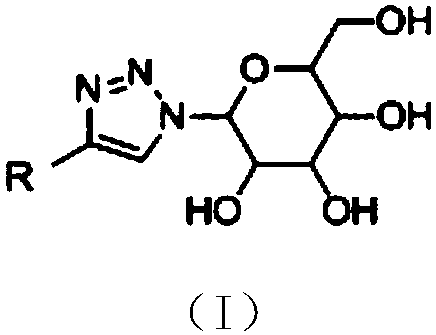
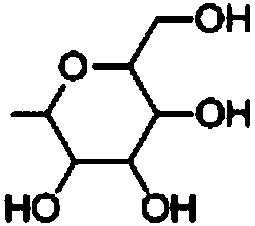
Application of saccharide coupled-1, 2, 3-triazole substituted polycyclic aromatic hydrocarbon derivative in preparation of anti-cancer drug
A polycyclic aromatic hydrocarbon, anticancer drug technology, applied in the field of biopharmaceutical chemical synthesis, can solve problems such as severe cardiotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] 1) Preparation of alkynyl-substituted polycyclic aromatic hydrocarbon raw materials
[0059]
[0060] 2) Preparation of target derivatives of the present invention
[0061]
[0062] In general formula 1), starting from commercially available raw material brominated polycyclic aromatic hydrocarbons, according to the aromatic alkynylation method and conditions reported in the literature (Angewandte Chemie-International Edition, 2005, vol.44, #39p.6362-6366 ; Chemistry-A European Journal, 1999, vol.5, #11p.3366-3381; Journal of Organic Chemistry, 1999, vol.64, #8p.2704-2710), can successfully prepare alkynyl-substituted polycyclic aromatic hydrocarbons.
[0063] The coupling reaction between brominated polycyclic aromatic hydrocarbons and trimethylethynyl silane is generally carried out in the presence of metal palladium catalysts and copper salts. The metal palladium catalysts that can be used include tetrakis (triphenylphosphine) palladium, bistriphenylphosphine P...
Embodiment 1
[0074]
[0075] The preparation method of compound I-1: comprises the following steps:
[0076]
[0077] (1) Synthesis of Compound II-1
[0078] Bromocorannene pale yellow solid (198.2 mg), bistriphenylphosphine palladium dichloride (19.6 mg), cuprous iodide (5.1 mg), trimethylethynyl silicon (153.0 mg) were dissolved in dry THF solution (10mL) and triethylamine (5mL) were stirred at 70°C for 3h. After the reaction was completed, 10% hydrochloric acid (3×10mL) was added to the reaction solution, then extracted with ethyl acetate (25mL), washed with distilled water (1×50mL), saturated brine (1×50mL), and then washed with After drying over anhydrous sodium sulfate, the solvent was evaporated to dryness with a rotary evaporator, and the obtained crude product was purified by silica gel column chromatography (mobile phase = 100% double-distilled petroleum ether) to obtain 139.1 mg of a yellow solid. The obtained solid (116.0 mg) and potassium carbonate (9.4 mg) were dissol...
Embodiment 2
[0084]
[0085] The preparation method of compound 1-2, comprises the following steps
[0086]
[0087] Compound II-1 (50.0 mg) and compound III-2 (75.0 mg) (compound III-2 can be purchased commercially, or prepared by a synthetic method reported in the literature. Reference Chemistry-A European Journal, 2013, vol.19 , #45p.15346-15357) was added to a mixture of tert-butanol and water (4mL) with a volume ratio of 1:1, and then copper sulfate pentahydrate (23.0mg) and sodium ascorbate (73.0mg) were added in one go. Stir at 90°C for 24h. After the reaction was completed, 25 mL of dichloromethane was added to the reaction solution, and then washed with distilled water (1×50 mL), the aqueous phase was extracted with dichloromethane (2×25 mL), the organic phases were combined, and then dried over anhydrous sodium sulfate. The solvent was evaporated to dryness with a rotary evaporator, and the obtained crude product was purified by silica gel column chromatography (petroleum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com