A solvothermal preparation method of lithium iron phosphate cathode material assembled into 3D flower-like two-dimensional nanosheets
A two-dimensional nano-lithium iron phosphate technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of low ion diffusion rate, poor rate discharge capability, and low electronic conductivity , to achieve the effect of simple preparation process route, improved rate performance and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Grind 5.56g of ferrous sulfate heptahydrate and 2.30g of ammonium dihydrogen phosphate, mix and dissolve in 50ml of ethylene glycol solvent, place in a 100mL beaker, put in a stirring magnet, heat up to 60°C and heat for 0.5 hours. Then add 0.630g of lithium carbonate and 10.5g of citric acid monohydrate into the container, and keep stirring at a constant temperature for 3h; As a surfactant, keep warm at 180°C. After 12 hours, the reaction was completed and cooled to room temperature. After repeated centrifugation and washing, the product was transferred to a vacuum drying oven at 120° C. for 6 hours to obtain a lithium iron phosphate cathode material with uniform particle size.
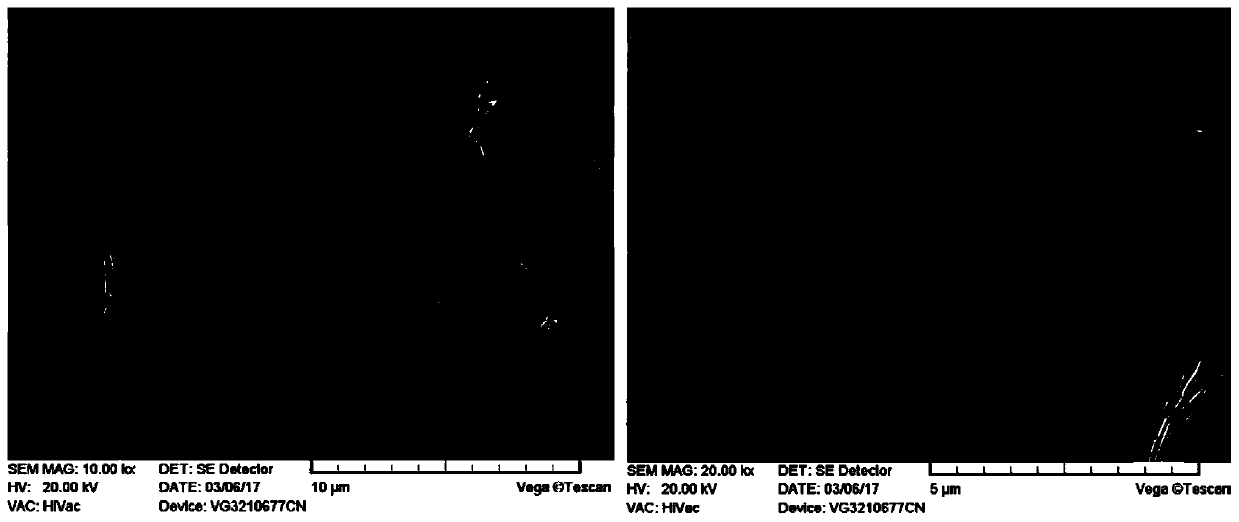
[0033] figure 1 It is a scanning electron microscope photo of the obtained lithium iron phosphate, and the synthetic material is a 3D flower-like morphology with holes assembled by two-dimensional nanosheets.
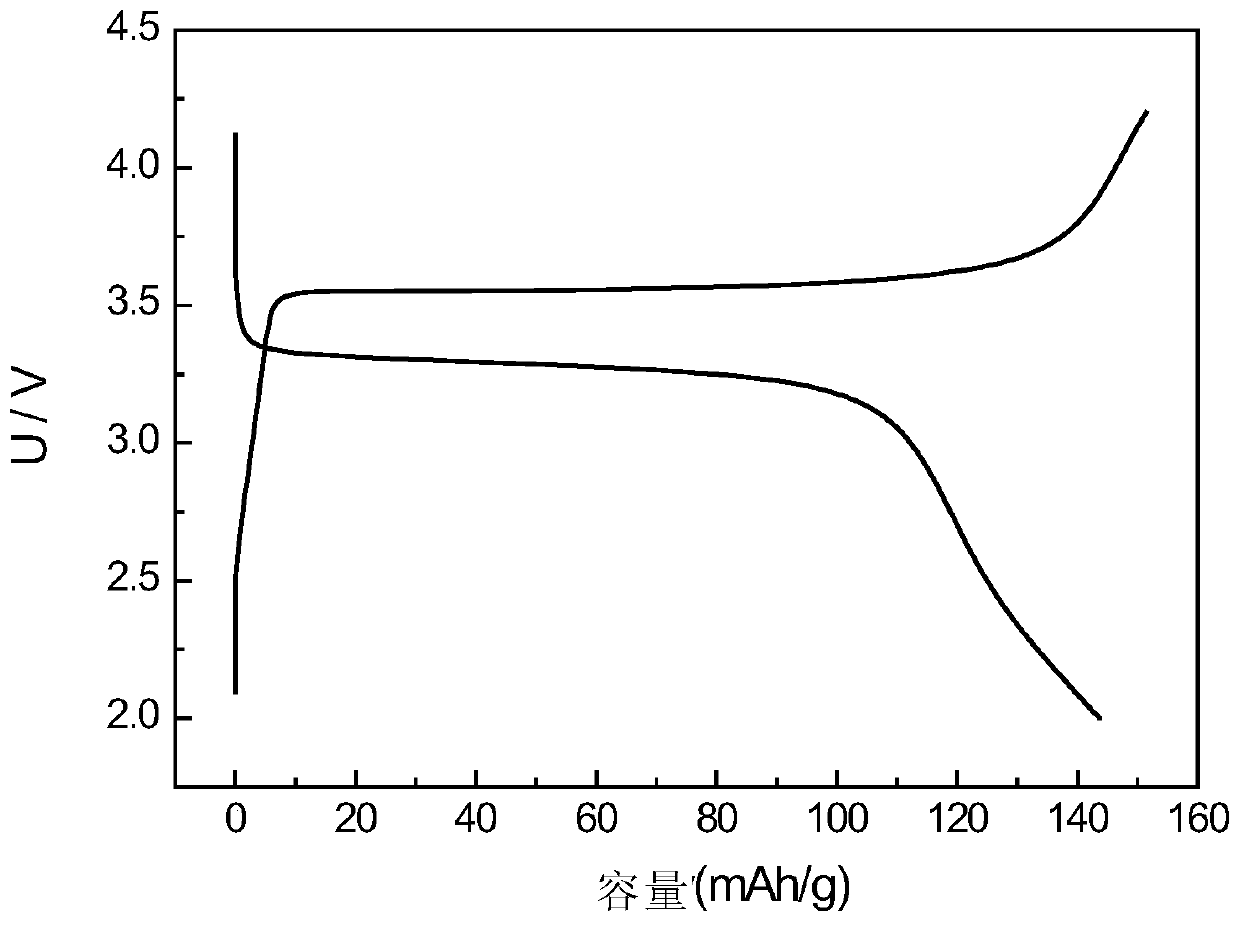
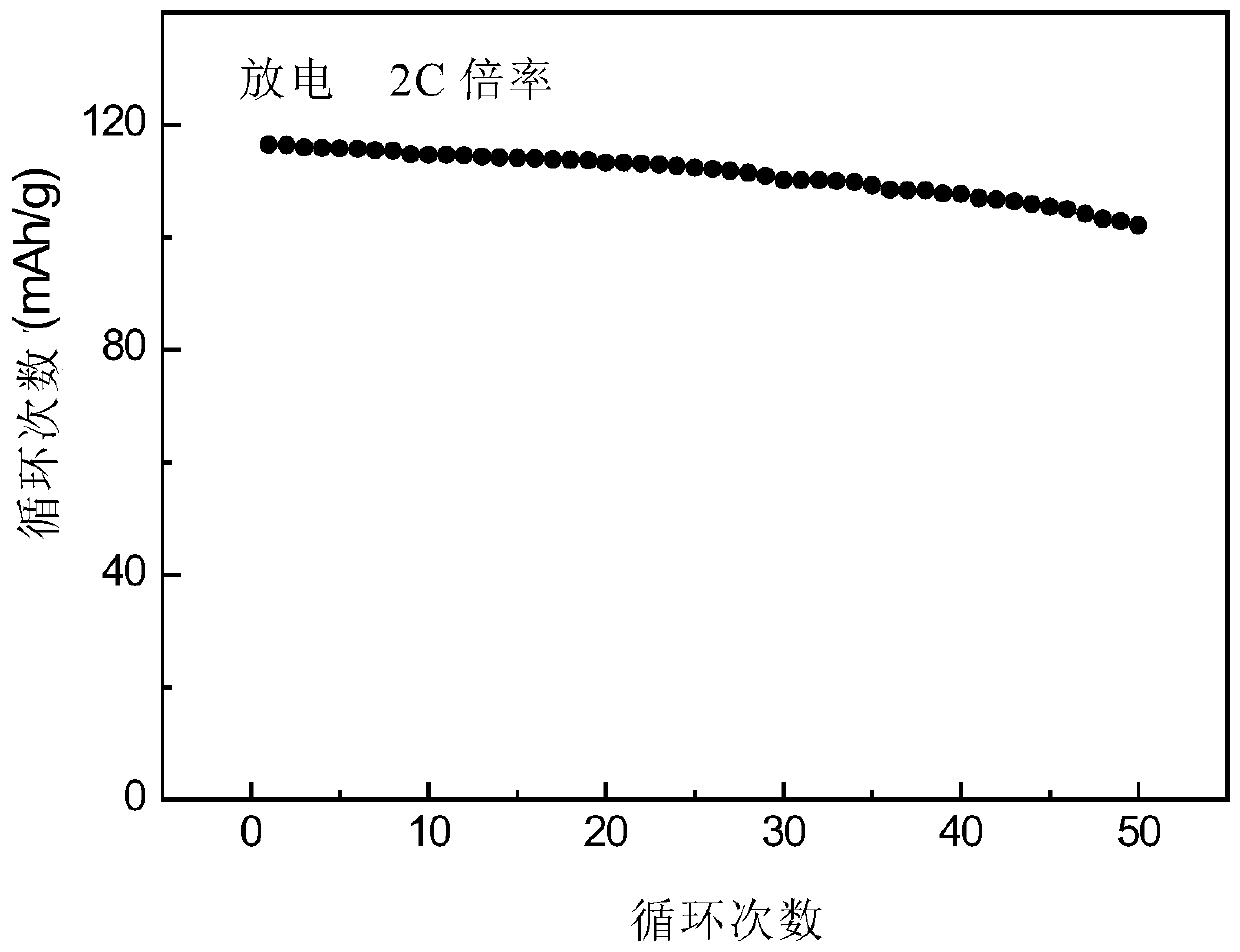
[0034] The electrochemical performance test of the obtained samples was carried ...
Embodiment 2
[0036] Grind 5.56g of ferrous sulfate heptahydrate and 2.30g of ammonium dihydrogen phosphate, mix and dissolve in 50ml of ethylene glycol solvent, place in a 100mL beaker, put in a stirring magnet, heat up to 60°C and heat for 0.5 hours. Then add 0.630g of lithium carbonate and 10.5g of citric acid monohydrate into the container, and keep stirring at a constant temperature for 3h; As a surfactant, keep warm at 180°C. After 12 hours, the reaction was completed and cooled to room temperature. After repeated centrifugation and washing, the product was transferred to a vacuum drying oven at 120° C. for 6 hours to obtain a lithium iron phosphate cathode material with uniform particle size.
[0037] The electrochemical performance was tested according to the method of Example 1, and the first-cycle reversible capacity of the obtained positive electrode material was 138mAh / g.
Embodiment 3
[0039]Grind 5.56g of ferrous sulfate heptahydrate and 2.30g of ammonium dihydrogen phosphate, mix and dissolve in 50ml of ethylene glycol solvent, place in a 100mL beaker, put in a stirring magnet, heat up to 60°C and heat for 0.5 hours. Then add 0.630g of lithium carbonate and 10.5g of citric acid monohydrate into the container, and keep stirring at a constant temperature for 3h; As a surfactant, keep warm at 180°C; continue for 12 hours, and cool to room temperature after the reaction. After repeated centrifugation and washing, the product was transferred to a vacuum drying oven at 120° C. for 6 hours to obtain a lithium iron phosphate cathode material with uniform particle size.
[0040] The electrochemical performance was tested according to the method of Example 1, and the first-cycle reversible capacity of the obtained positive electrode material was 136mAh / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com