Compound medicine as well as preparation method and application thereof
A compound and drug-based technology, which is applied in the field of medicine, can solve problems such as ineffective phosphorus reduction and gastrointestinal discomfort, and achieve the effects of avoiding gastrointestinal adverse reactions, good targeted curative effect, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1). Micronize 1g of lanthanum carbonate powder, add it to 10-25g of ethyl acetate solution, and disperse it ultrasonically. The ultrasonic power is 100-200W, and the dispersion time is 30min;
[0036] (2). Dissolve 1.5g PLGA in 100-300g ethyl acetate solution, heat and stir to mix evenly to form a solution;
[0037] (3). The mixture obtained in (1) is uniformly dispersed in the solution described in (2), heated, stirred and mixed to form a primary emulsion;
[0038](4). Dissolve 0.6g of polyvinyl alcohol in 100-300mg of water, then add 1.5-5g of NaCl, heat and mix to form a polyvinyl alcohol solution;
[0039] (5). Add the emulsion formed in (3) into the polyvinyl alcohol solution described in (4), stir mechanically at a speed of 4500r·min-1, and stir for 6h to form a S / O / W type double emulsion.
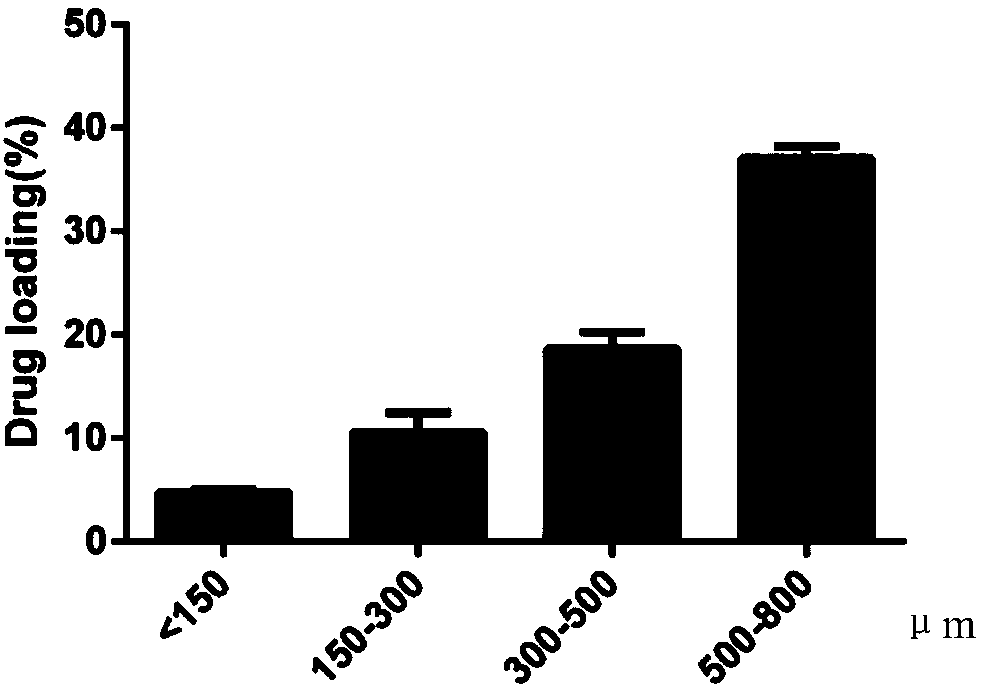
[0040] (6). Stir the emulsion obtained in (5) to volatilize ethyl acetate, solidify into balls, and then centrifuge, wash, and dry to obtain the PLGA drug-loaded microsphere...
Embodiment 2
[0042] Preparation of compound drugs:
[0043] (1). Micronize 0.5g of lanthanum carbonate powder, add it to 1-3ml of ethyl acetate solution, and disperse it ultrasonically. The ultrasonic power is 100-200W, and the dispersion time is 30min;
[0044] (2). Dissolve 1g PLA in 12-40ml ethyl acetate solution, heat and stir to mix evenly to form a solution;
[0045] (3). The mixture obtained in (1) is uniformly dispersed in the solution described in (2), heated, stirred and mixed to form a primary emulsion;
[0046] (4). Dissolve 0.8g of polyvinyl alcohol in 100-300mL of water, then add 1.5-5g of NaCl, heat and mix to form a polyvinyl alcohol solution;
[0047] (5). Then add the emulsion formed in (3) into the polyvinyl alcohol solution described in (4), stir mechanically at a speed of 4500r min-1, and stir for 6h to form a S / O / W complex
[0048] (6). Stir the emulsion obtained in (5) to volatilize ethyl acetate, solidify into balls, and then centrifuge, wash, and dry to obtain th...
Embodiment 3
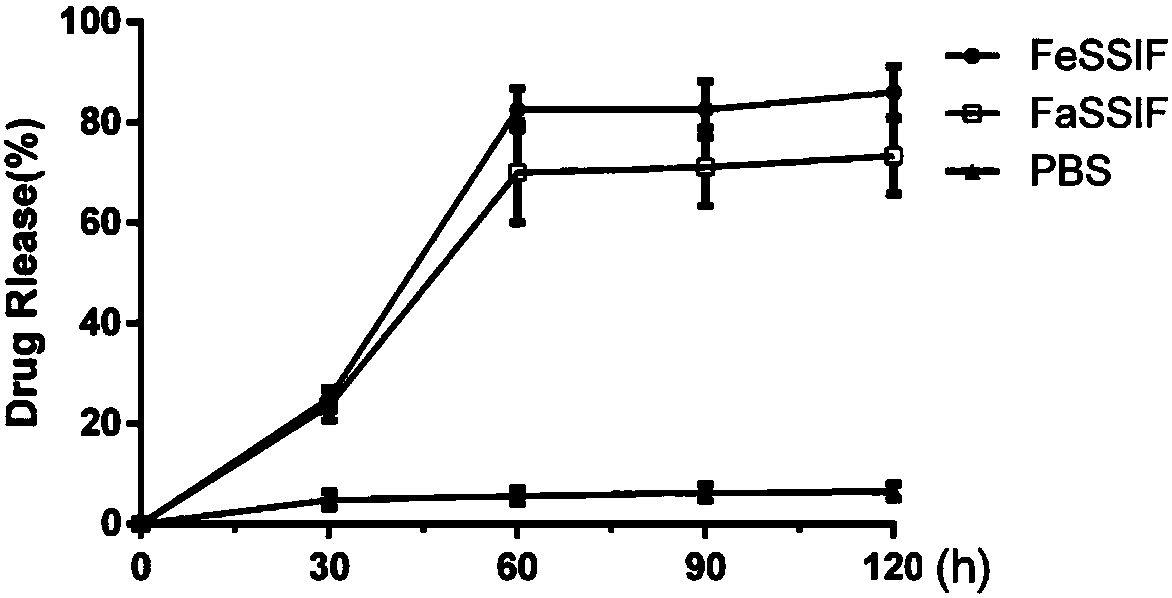
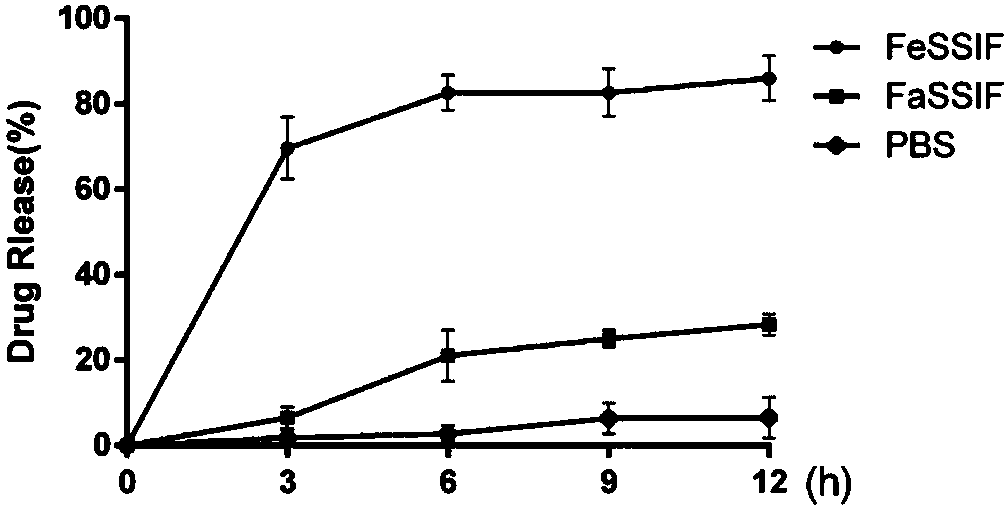
[0050] This example investigates the protective effect of PLGA on lanthanum carbonate in an acidic environment.
[0051] Group A is mixed with 500mg of lanthanum carbonate powder (minimum dosage for patients with mild symptoms, up to 3.75g at one time) and 500ml of dilute hydrochloric acid with pH=2 (a person can secrete 500ml of gastric juice in one meal),
[0052] Group B was mixed with PLGA-wrapped composite dosage form containing the same amount of lanthanum carbonate and 500ml of dilute hydrochloric acid with pH = 2. After 4 hours, it was found that the pH of the liquid in group A was equal to 7, and the lanthanum carbonate powder precipitated at the bottom of the beaker without obvious Change; the PH value of the liquid in group B hardly rose, and powder settled at the bottom of the beaker, and its amount was more than that in group A.
[0053] Thereby, explain that lanthanum carbonate powder is dissolved in acid, and PLGA has played protective effect to lanthanum carbon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com