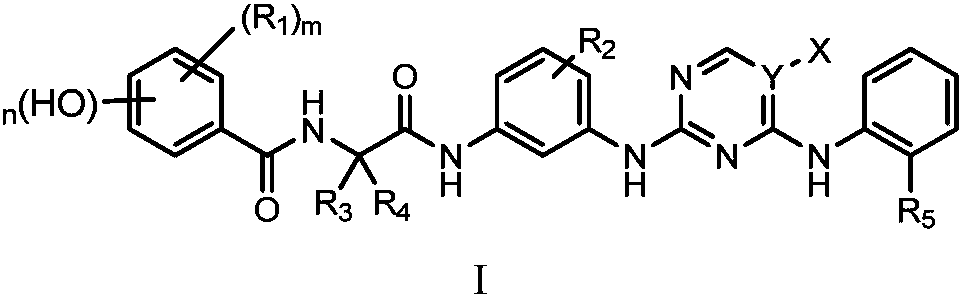
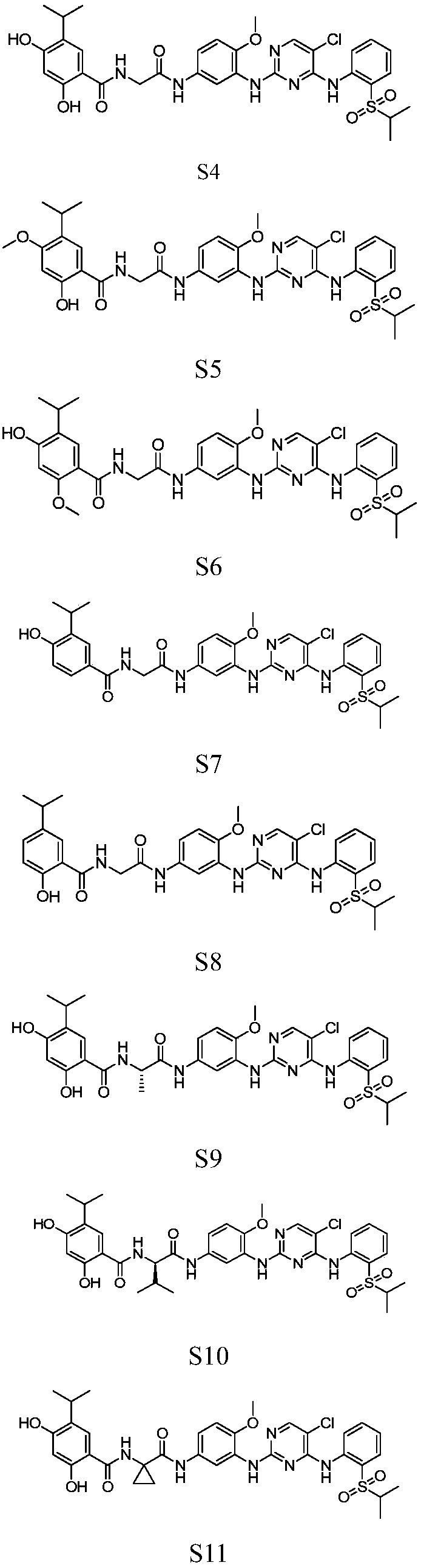
2, 4-diaminopyrimidine compound containing phenol fragment, preparation method, pharmaceutical compositions and use thereof
A technology of diaminopyrimidine and compound, which is applied to a class of 2,4-diaminopyrimidine compounds containing phenol fragments, their preparation, pharmaceutical compositions and application fields, can solve problems such as low activity, and achieve enhanced binding effect , Change the acidity and alkalinity, increase the effect of the site of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
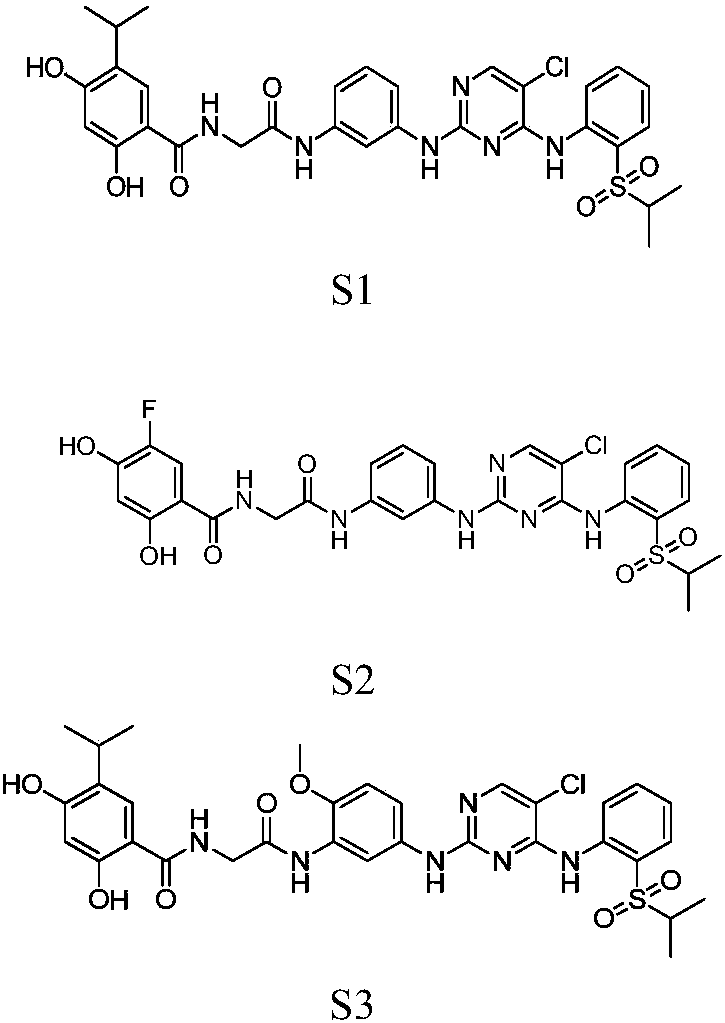
[0056] Synthesis of Preparation Example 1 Compound S1
[0057]
[0058] Synthesis of intermediates 1-3:
[0059] Dissolve 1.2eq Boc-glycine (1-1) in 50ml of dry dichloromethane, add 2eq 2-(7-azobenzotriazole)-N,N,N',N' successively under ice-cooling -tetramethyluronium hexafluorophosphate (HATU), 2eq N-hydroxy-7-azabenzotriazole (HOAT), 1eq intermediate 1-2 and 5eq N,N-diisopropylethylamine ( DIPEA), raised to room temperature and stirred overnight, spin-dried dichloromethane, added ethyl acetate, extracted with 1N HCl, combined organic layers, washed with saturated sodium bicarbonate and sodium chloride successively, and dried over anhydrous sodium sulfate. Filtrate, spin dry and put on the column, petroleum ether: ethyl acetate = 2:1, the product 1-3 was obtained.
[0060] Synthesis of Intermediates 1-4:
[0061] Dissolve intermediate 1-3 in methanol, add 0.2eq palladium carbon (10% palladium content), and react overnight at room temperature after hydrogen replacement....
preparation Embodiment 2
[0076] Synthesis of Preparation Example 2 Compound S2
[0077]
[0078] The synthesis of compound S2 was the same as that of S1 except that intermediate 2-1 was used instead of intermediate 1-7.
[0079] 1 H NMR (300MHz, Methanol-d4) δ8.56(d, J=8.3Hz, 1H), 8.17(s, 1H), 8.06(s, 1H), 7.79(d, 1H), 7.65(d, J= 12.4Hz, 1H), 7.56(t, J=7.6Hz, 1H), 7.28–7.16(m, 3H), 7.06(t, J=7.5Hz, 1H), 6.46(s, 1H), 4.58(s, 2H), 3.36–3.33(m, 1H), 1.26(d, J=7.1Hz, 6H).
[0080] Synthesis of Intermediate 2-1:
[0081]
[0082] Synthesis of Intermediate 2-1-2:
[0083] Dissolve 1eq of raw material 2-1-1 in boron trifluoride ether solution, add 4eq of acetic acid, and reflux at 95°C for 3 hours under nitrogen protection. After the reaction is complete, add saturated sodium carbonate solution to quench, extract with ethyl acetate, and combine The organic layer was washed successively with saturated sodium bicarbonate and sodium chloride, and dried over anhydrous sodium sulfate. Filtrate, spin d...
preparation Embodiment 3
[0088] Synthesis of Preparation Example 3 Compound S3
[0089]
[0090] The synthesis of compound S3 was the same as that of S1 except that intermediate 3-1 was used instead of m-nitroaniline.
[0091] 1 H NMR (300MHz, Methanol-d4) δ8.56 (d, J = 8.5Hz, 1H), 8.14 (d, J = 2.5Hz, 1H), 7.81 (d, 1H), 7.61 (s, 1H), 7.55 (t, J=7.8Hz, 1H), 7.25(t, J=7.7Hz, 1H), 7.04(d, J=8.5Hz, 1H), 6.78(d, J=8.6Hz, 1H), 6.31(s ,1H), 4.59(s,3H), 4.17(s,2H), 3.27–3.12(m,2H), 1.22(d,J=6.9Hz,12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com