Preparation method of larotrectinib intermediate
A technology for latrextinib and intermediates, which is applied in the field of preparation of latrextinib intermediates, can solve the problems of unfavorable industrial production, numerous steps, and reduced atomic economy of the preparation process, so as to improve the reaction yield and operational reliability. controllability, facilitate development, and reduce the effect of noble metal catalysts and other expensive reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under nitrogen atmosphere, add (7aS,12R)-12-phenyl-7a,8,9,10-tetrahydro-12H-naphthol[1,2-e]pyrrolo[2,1- b] [1,3]oxazine (II) (3.0g, 10mmol) and 30mL of dry tetrahydrofuran, cooled to 0 ° C, dropwise added 2,5-difluorophenylmagnesium bromide (III) (3.2g, 15mmol ) in THF solution 30mL. Raise the temperature to 25-35° C., keep the temperature and stir the reaction for 3-5 hours, and TLC detects that the reaction is complete. The reaction was quenched with 25 mL of saturated ammonium chloride, extracted three times with dichloromethane, the organic phases were combined, washed with water and saturated brine in sequence, and dried over anhydrous sodium sulfate. Concentrate, and recrystallize the residue with ethyl acetate to obtain off-white solid 1-[(R)-[(2R)-2-(2,5-difluorophenyl)-1-tetrahydropyrrolidinyl]phenyl Methyl]-2-naphthol (IV) 3.5g, yield 84.3%, FAB-MS m / z: 416[M+H] + .
Embodiment 2
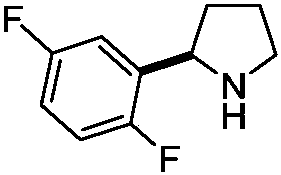
[0027] Add 1-[(R)-[(2R)-2-(2,5-difluorophenyl)-1-tetrahydropyrrolidinyl]phenylmethyl]-2-naphthol ( IV) (2.1g, 5mmol), 10% palladium on carbon (Pd / C) (0.32g, 0.3mmol) and methanol 25mL, hydrogen gas was passed through at room temperature and normal pressure until hydrogen gas was no longer absorbed, and hydrogen gas flow was stopped. The catalyst was recovered by filtration, and the solvent was recovered under reduced pressure to obtain 0.79 g of reddish oil (R)-2-(2,5-difluorophenyl)tetrahydropyrrolidine (I), with a yield of 86.3%; 1H NMR (CDCl 3 )δ7.24(m, 1H), 6.94(m, 1H), 6.85(m, 1H), 4.40(t, J=7.6Hz, 1H), 3.16(m, 1H), 3.04(m, 1H), 2.21-2.30(m, 1H), 1.77-1.95(m, 3H), 1.57-1.67(m, 1H); EI-MS m / z: 184[M+H] + .
Embodiment 3
[0029] Add 1-[(R)-[(2R)-2-(2,5-difluorophenyl)-1-tetrahydropyrrolidinyl]phenylmethyl]-2-naphthol (IV ) (2.1g, 3mmol), ceric ammonium nitrate (4.1g, 7.5mmol) and 60mL of a mixed solvent of acetonitrile and water (volume ratio 2:1), stirred at room temperature for 5-7 hours, and TLC detected that the reaction was complete. Filtrate, add sodium bicarbonate solution to the filtrate, and extract three times with dichloromethane, combine the organic phases, and dry over anhydrous sodium sulfate. Concentration under reduced pressure gave 0.76 g of reddish oil (R)-2-(2,5-difluorophenyl)tetrahydropyrrolidine (I), yield 83.0%; 1H NMR (CDCl 3 )δ7.24(m, 1H), 6.94(m, 1H), 6.85(m, 1H), 4.40(t, J=7.6Hz, 1H), 3.16(m, 1H), 3.04(m, 1H), 2.21-2.30(m, 1H), 1.77-1.95(m, 3H), 1.57-1.67(m, 1H); EI-MSm / z: 184[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com