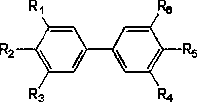
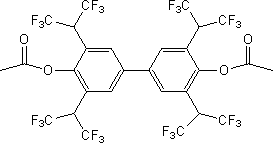
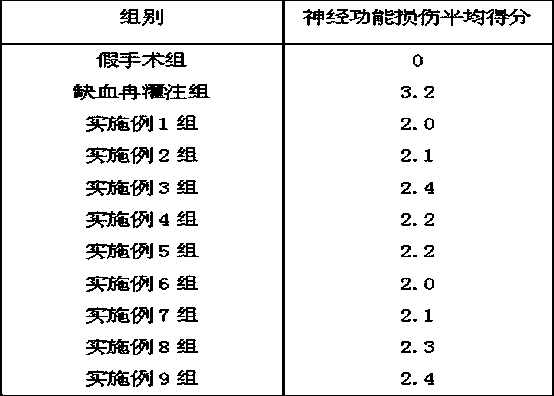
Biphenyl derivative and preparation method and medical application thereof
A derivative, biphenyl technology, applied in the field of medicine, can solve problems such as limiting clinical application, achieve good therapeutic effect, accurately control ischemia and reperfusion time, and reduce the consumption of vitality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the preparation of compound I-1
[0057]
[0058] Compound I-1
[0059] 10.0 g of 4'-benzyloxy-3,3',5,5'-tetrakis(1,1,1,3,3,3-hexafluoroisopropyl)biphenyl-4-acetate at room temperature Dissolve in 200mL of methanol, then add 1.0g of 10% palladium carbon, vacuumize, pass through hydrogen, repeat three times, seal and react at room temperature for 17h, filter palladium carbon in the reaction solution, and concentrate the filtrate to dryness. The residue was separated by HPLC to obtain 1.7 g of compound (I-1).
Embodiment 2
[0060] Embodiment 2: the preparation of compound 1-2
[0061]
[0062] Compound I-2
[0063] Add 5 g of 4,4'-dihydroxy-3,3',5,5'-tetrakis(1,1,1,3,3,3-hexafluoroisopropyl)biphenyl into 30 mL of acetic anhydride, Under the protection of nitrogen, after reflux for 3 hours, the reaction solution was cooled to room temperature, and acetic anhydride was removed under reduced pressure. The residue was separated by HPLC to obtain 1.2 g of compound (I-2).
Embodiment 3
[0064] Embodiment 3; The preparation of compound 1-3
[0065]
[0066] Compound I-3
[0067] (1) Take 5g of 4,4'-dihydroxy-3,3',5,5'-tetrakis(1,1,1,3,3,3-hexafluoroisopropyl)biphenyl with anhydrous THF (100 mL) was dissolved, and 2.24 g of NaOH as a solid and 8.185 g of bromochloromethane were added. in N2 Reflux for 2 hours under air protection, cool the reaction solution to room temperature, filter, concentrate, and the residue is separated by HPLC to obtain the intermediate.
[0068] (2) Add 14 mL of triethylamine and 0.5 mL of 85% phosphoric acid to 100 mL of anhydrous acetonitrile in sequence, and add the intermediate obtained in (1) into the acetonitrile solution under stirring conditions, and then react at 65° C. for 2 h. The reaction solution was cooled to room temperature, the solvent was evaporated, the residue was dissolved in 15 mL of water, and the pH was adjusted to 1.5 with 8M HCl; extracted with anhydrous ether, the organic phases were combined, washed wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com