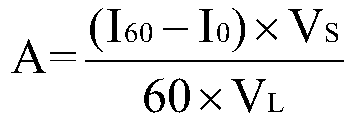A method for constructing high-efficiency secretion and expression of transpeptidase sortase A in Escherichia coli
A technique for secreting, expressing, and recombining Escherichia coli, which is applied in the field of genetic engineering and can solve the problems of time-consuming, cost, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0010] The method for the efficient secretion of Sortase A by recombinant escherichia coli is: inoculate the cultivated seeds (LB medium) into the 500mL Erlenmeyer flask with 2% inoculum size; the fermentation medium is TB medium (peptone 12.0 g / L, yeast powder 24.0g / L, glycerin 4.0g / L, KH 2 PO 4 2.31g / L, K 2 HPO 4 12.54g / L, pH 7.0); the fermentation conditions adopted are: fermentation temperature 30°C, induction culture 36h, inducer IPTG concentration 100mM / L, induction timing OD 600 = 0.6.
[0011] Sortase A purification method: After 36 hours of induction, the bacterial solution was centrifuged at 9000 rpm for 3 minutes at 4°C and the supernatant was taken, which was the crude enzyme solution. Sortase A in the crude enzyme solution was purified with His Tag-tagged protein chelating magnetic beads (Beaver Nano Technology Co., Ltd.). For specific steps, please refer to the instructions for use of the magnetic beads.
[0012] Determination of protein content: Use the B...
Embodiment 1
[0016] Embodiment 1 Realization of high-efficiency secretion and expression of Sortase A
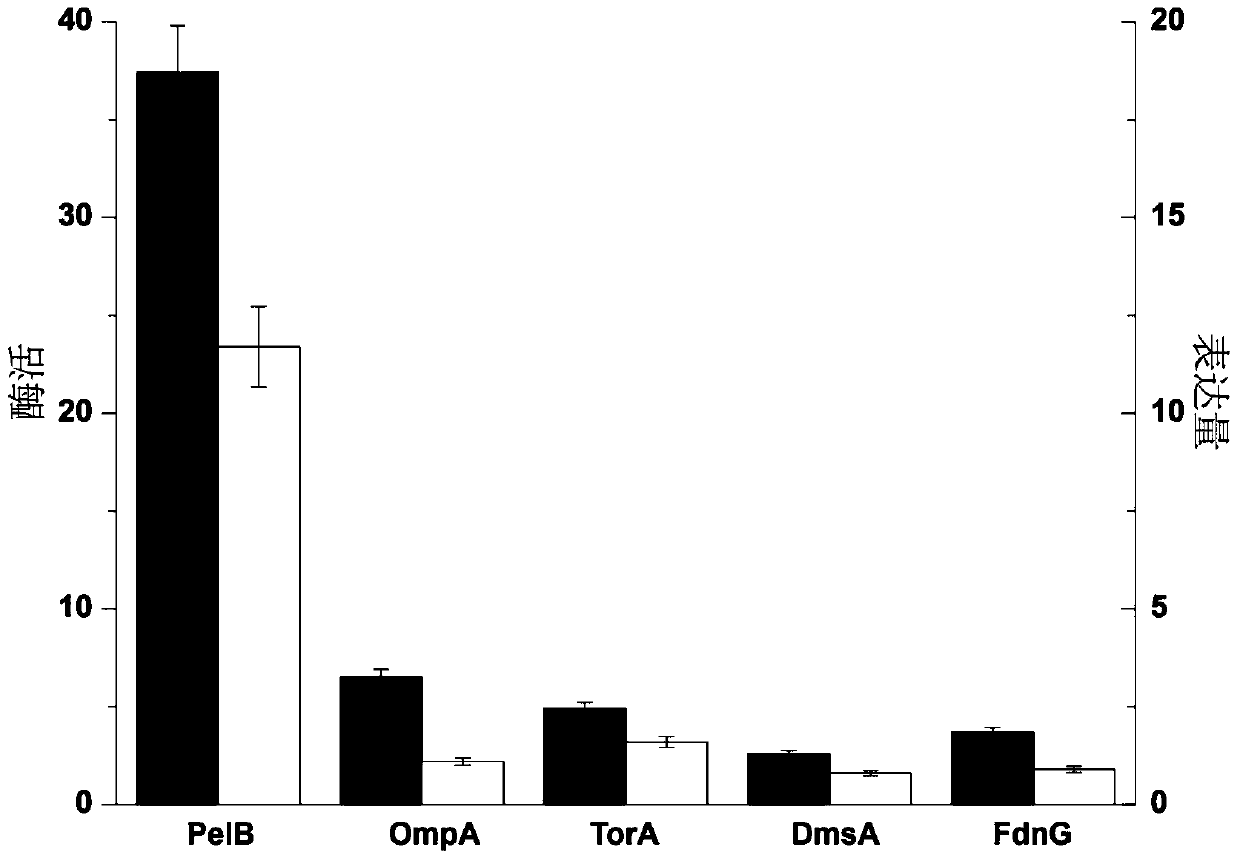
[0017] Using the genome of Staphylococcus aureus as a template, the srtA gene (SEQ ID NO.1) obtained by removing the N-terminal signal peptide was amplified with srt-F and srt-R (Table 1) primers, and cloned into the Nco I / Bam HI site to obtain the pET22a-PelB-srtA vector. Using the Escherichia coli genome as a template, OmpA was amplified with primers OmpA-F and OmpA-R, TorA-F and TorA-R, DmsA-F and DmsA-R, FdnG-F and FdnG-R (Table 1). , TorA, DmsA and FdnG four signal peptide coding genes, and use OmpA-SrtA-F and OmpA-SrtA-R, TorA-SrtA-F and TorA-SrtA-R, DmsA-SrtA-F and DmsA-SrtA- R, FdnG-SrtA-F and FdnG-SrtA--R (Table 1) primers amplified to the srtA gene fragment that could be fused with the four signal peptide coding genes. Using fusion PCR technology, four fragments of OmpA-SrtA, TorA-SrtA, DmsA-SrtA, and FdnG-SrtA were obtained, and the PelB signal peptide on the pET22a-PelB-sr...
Embodiment 2
[0020] Example 2 Co-expression of molecular chaperones improves the secreted expression level of Sortase A
[0021] Starting from the E.coli BL21(DE3) strain carrying the pET22a-PelB-srtA vector, after preparation of competence, five different molecular chaperone co-expression plasmids (pG-KJE8, pGro7, pKJE7, pG-Tf2 and pTf16 were transformed into To the bacterial strain. After the activation of five different molecular chaperone co-expressed bacterial strains, induce expression, detect the content and enzyme activity of extracellular Sortase A. The results (table 3) show that the effect of pG-Tf2 co-expression is the best, and the secretory expression of Sortase A The amount was further increased to 90.2mg / L, and the extracellular enzyme activity level was further increased to 34.2U / mL.
[0022] Table 1 Primers used in the construction of Sortase A secreting strains mediated by different signal peptides
[0023]
[0024]
[0025] Table 2 Primers used for Sortase A codon ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com