A kind of antibacterial protein and isolated nucleic acid, antibacterial drug and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
[0042] Constructing the expression vector containing the antibacterial protein nucleic acid comprises the following steps.
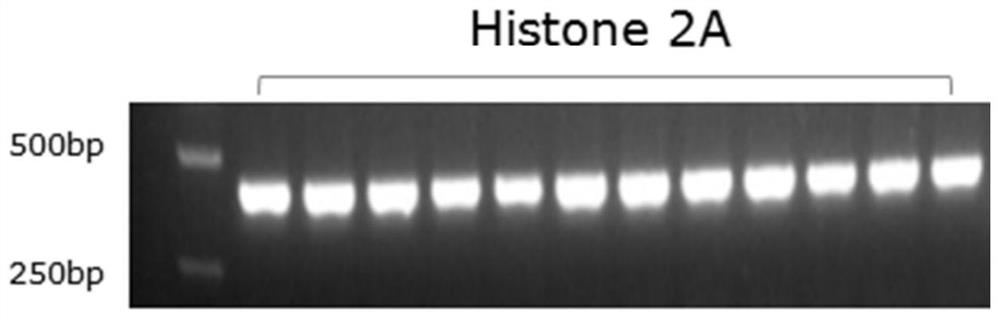
[0043] 1. Primer Design
[0044] RIP2 in wild zebrafish infected with E. silguris and in RIP2 - / - The differentially expressed histone H2A nucleic acid was screened in the transcriptome library of the knockout homozygous zebrafish, and a pair of specific primers were designed on this basis. The specific primers include upstream primers and downstream primers.
[0045] Wherein, the base sequence of the upstream primer is:
[0046] 5'-GTC AAGCTT AAGACCAAGATGAGCGGAA-3' (as shown in SEQ ID NO.25)
[0047] Wherein, the underlined AAGCTT is the restriction site of Hind III.
[0048] The base sequence of the above-mentioned downstream primers is:
[0049] 5'-GAT GGTACC TTGCCTTTGGCAGCCTTCTC-3' (as shown in SEQ ID NO.26)
[0050] Wherein, the underlined place GGTACC is the enzyme cleavage site of KPN I.
[0051] 2. PCR amplification
[0052] Using PCR...
no. 2 example
[0061] To verify the expression of histone H2A-V2 and histone H2A-V8 in fish somatic cells.
[0062] In this experimental example, the expression of histone H2A-V2 and histone H2A-V8 in fish somatic cells was examined by Western blot (Western blotting). The specific operation of Western blot is as follows:
[0063] 1. Collect the target protein
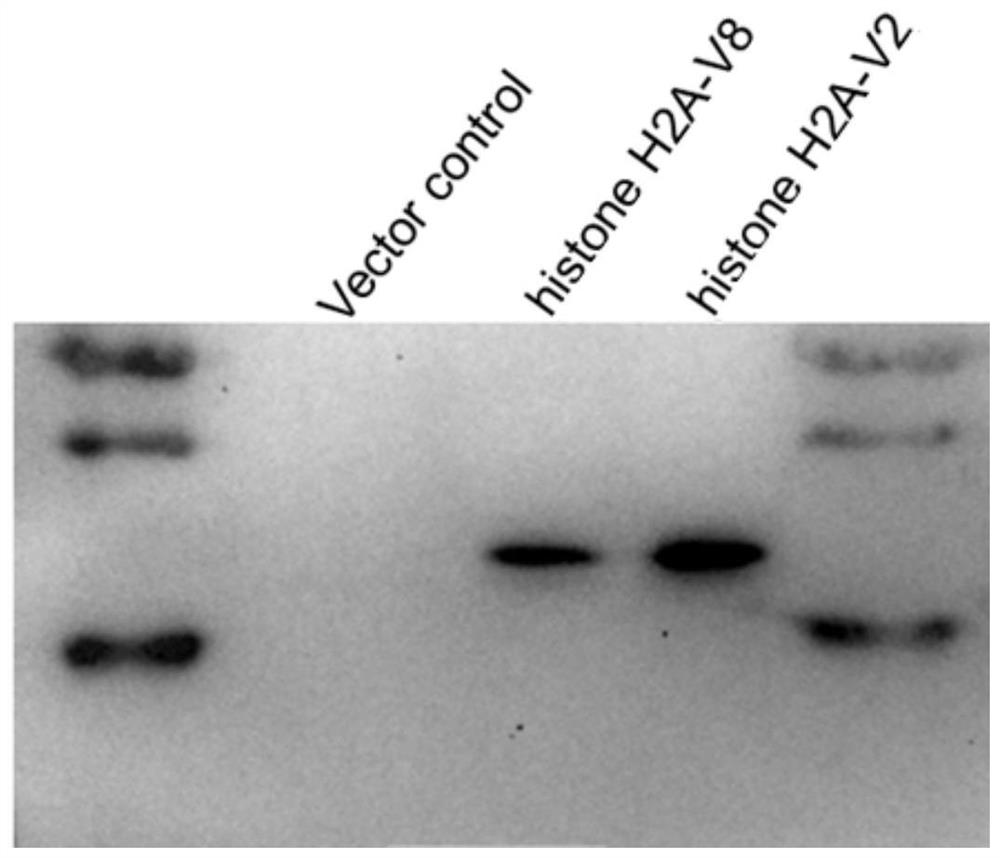
[0064] The histone H2A-V2 and histone H2A-V8 plasmids constructed in the first example were transfected into fish EPC cells, and the target protein was extracted after 48 hours.
[0065] 2. Electrophoresis
[0066] Prepare a 12% SDS-PAGE gel, add 20 μl of the target protein, and conduct electrophoresis at 80v and then at 120v.
[0067] 3. Transfer film
[0068] Transfer the target protein to PVDF membrane, 30v constant pressure transfer membrane for 90 minutes, 5% skimmed milk powder (TBST preparation) to block for one hour, then add mouse anti-monoclonal FLAG tag antibody (Sigma company, 1:5000 dilution) and incubate at 4°C Over...
no. 3 example
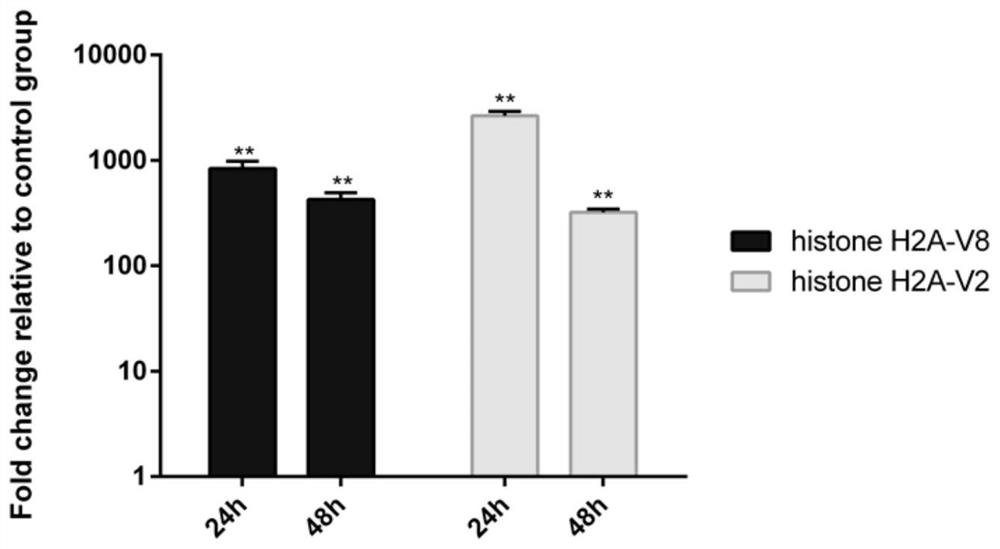
[0073] Validation of expression of histone H2A-V2 and histone H2A-V8 in fish infected with E. caturis.
[0074] In this experimental example, qRT-PCR was used to test the recombinant expression of histone H2A-V2 and histone H2A-V8 plasmids in fish. The specific operations are as follows:
[0075] First, the plasmids of histone H2A-V2 and histone H2A-V8 provided in the first embodiment were microinjected into zebrafish embryos by microinjection. Then, on the fourth day after fertilization, after the zebrafish larvae were fully hatched, the plasmid groups injected with the empty plasmid group, histone H2A-V2 and histone H2A-V8 were divided into groups for soaking infection, and the infection concentration was 2 ×10 8 pfu (plaque forming unit) / ml. 3 replicates per group, 30 fish per replicate. At 24h and 48h after infection, 10 juvenile fish were taken from each group, washed with PBS, and then homogenized with Trizol. Then, the RNA of the 3 groups of samples was extracted an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com