A conjugate containing diclofenac, a preparing method thereof and uses of the conjugate
A technology of diclofenac and conjugates, which is applied in the field of natural medicine and drug therapy, can solve the problems of poor solubility, low bioavailability, weak anti-tumor activity of GAA, etc., so as to overcome poor solubility, remove cancer cells, and increase anti-cancer drugs active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
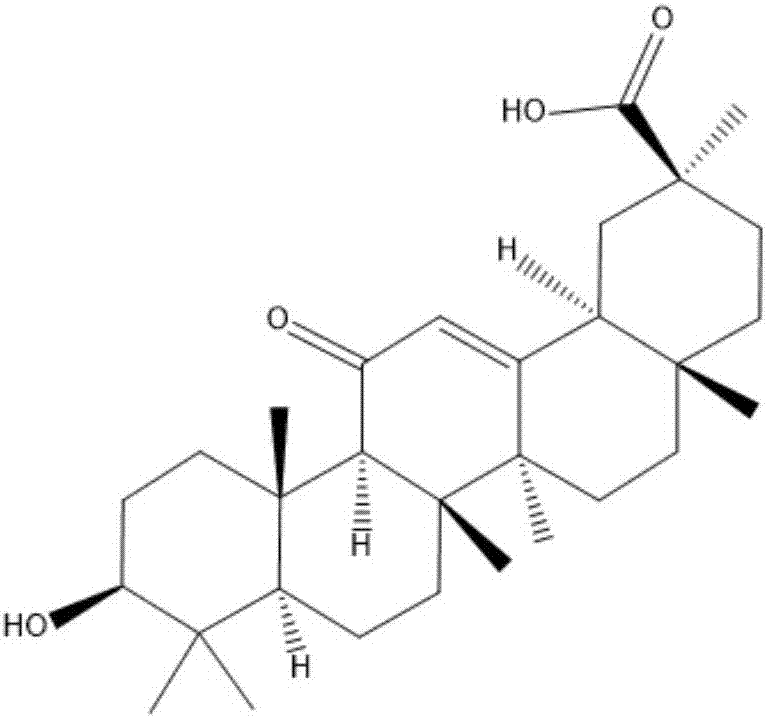
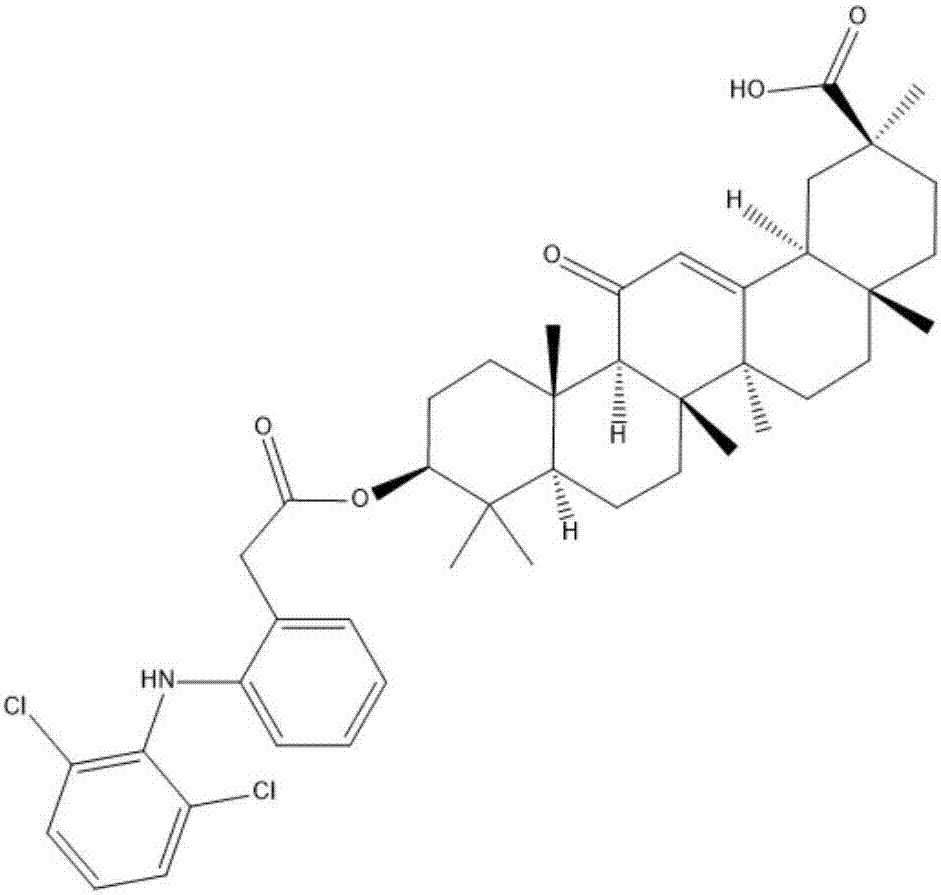
[0026] Glycyrrhetinic acid (1.1mmole) and diclofenac (1.3eq) were dissolved in anhydrous dichloromethane (30ml), under the catalysis of pyridine (0.5g) and 4-dimethylaminopyridine (0.5g), room temperature 25- React overnight at 30°C, filter, pass through a silica gel chromatography column (the elution solvent used is ethyl acetate and petroleum ether in a volume ratio (3-8):7), rotary evaporate, and dry to obtain the target compound as a white solid. Through liquid chromatography mass spectrometry and NMR analysis, the newly prepared white solid target compound is a conjugate with the structure shown in formula (I):
[0027]
Embodiment 2
[0029] In vitro anti-tumor effect evaluation of target compounds. In this example, breast cancer MCF-7, lymphoma BC-3 and liver cancer HepG2 cells were used to evaluate their efficacy, while LO2 liver cells were used to detect their toxicity.
[0030] Take the cells in the logarithmic growth phase and inoculate 4-40×10 cells according to the size of the cells 3 Each was placed on a 96-well plate, and after 24 hours of growth, the supernatant was discarded, and then administered in groups as follows: Tumor cells were set up with no drug addition group and drug addition group (concentration 1-100 μM for tumor cells, concentration 5-200 μM for LO2 cells), glycyrrhetinic acid coupled to diclofenac GAA-DC (i.e. the target compound obtained in Example 1), glycyrrhetinic acid GAA, diclofenac DC and an equimolar mixture of the two GAA+DC. Set up 4 to 6 multiple wells in each group, culture for 72 hours, discard the supernatant, add 100 μl of MTT (tetrazolium salt) serum-free culture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com