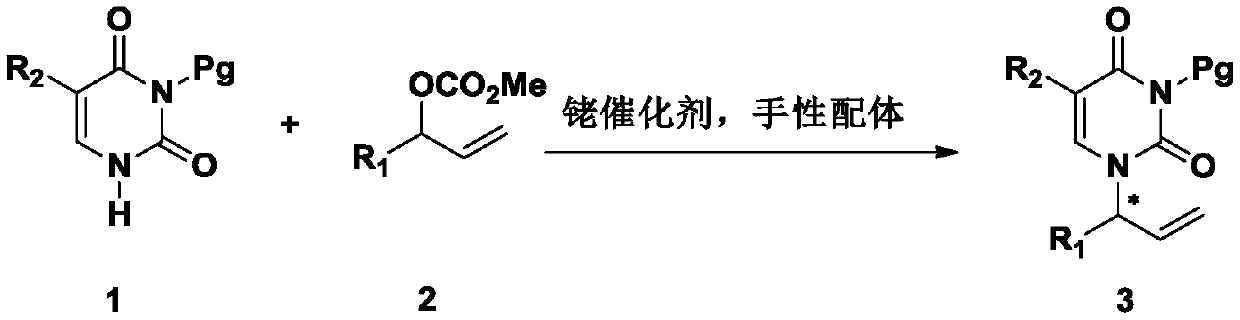
A kind of asymmetric allylation reaction to synthesize chiral n 1 - Allylpyrimidine method
An allylpyrimidine and allylation technology, which is applied in the field of asymmetric synthesis in organic chemistry, can solve the problems of difficult preparation of chiral substrates and high cost, and achieves abundant product structures, efficient synthesis methods, and product stereoselectivity. high sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019]
[0020]
[0021]
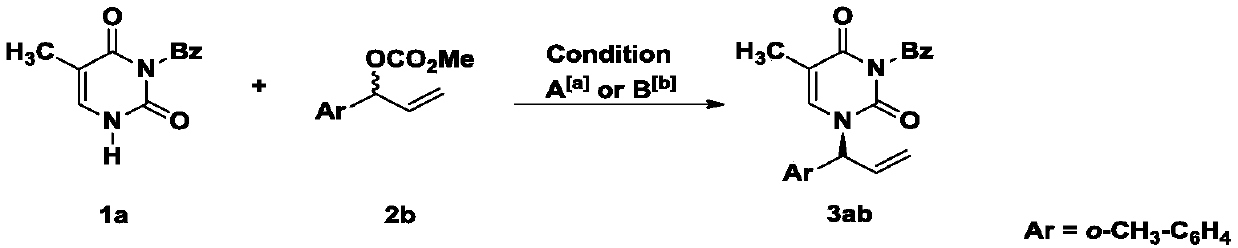
[0022] a Entry 1, Conditions A: 1a(0.2mmol, 1.0equiv), cinnamyl carbonate 2b(0.2mmol) [Ir(COD)Cl] 2 (2mol%) and L1 (4mol%), tetrahydrofuran (THF) (0.2mL), K 3 PO 4 (1.0equiv.) 50℃,N 2 ,12h. b Entries 2-10, Conditions B: 1a (0.2mmol, 1.0equiv), cinnamyl carbonate 2b (0.2mmol)[Rh(COD)Cl] 2 (3mol%) and Ligand(6mol%), DCE(0.2M), 80℃, N 2 ,12h. c The ratio determined form crude 1 H NMR. d Yields of isolated product. e The ee values were determined by chrial-phase HPLC analysis.
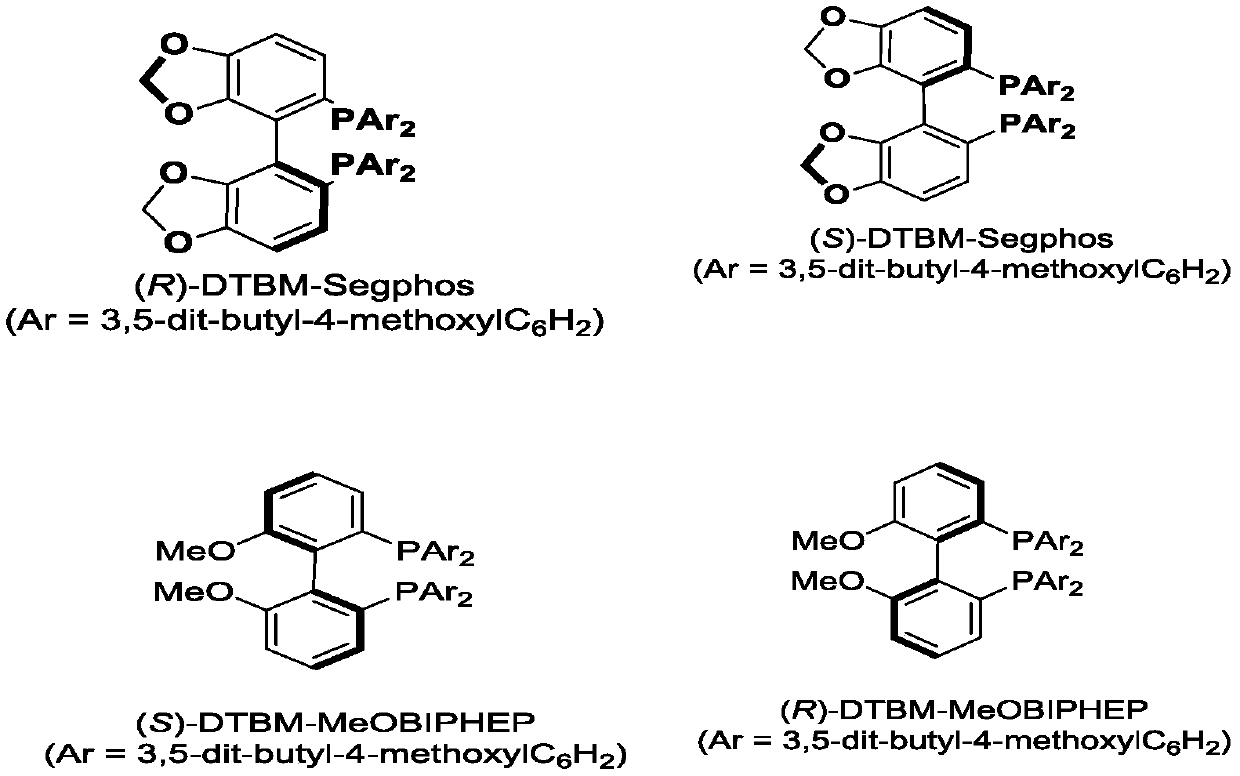
[0023] In the screening process of reaction conditions, the effects of metal Ir and metal Rh on the reaction were first investigated (entries1-2). At the same time, by comparing the effects of different ligands on the reaction, the ligands L9 and L10 were determined to be the best ligands.
[0024] Investigation of reaction conditions: In a 10mL vacuum tube, add N 3 -Bz protected thymine (46.1mg, 0.2mmol), [Rh(COD)Cl 2 ] (2.9 mg, 0.006 mmo...
Embodiment 2
[0039] In a 10 mL vacuum tube, add N 3 -Bz protected thymine (46.1mg, 0.2mmol), [Rh(COD)Cl 2 ] (2.9 mg, 0.006 mmol) and (R)-DTBM-Segphos (14.2 mg, 0.012 mmol). The reaction tube was filled with nitrogen by nitrogen replacement 3 times, and then, under nitrogen flow, carbonate 2b (82.4 mg, 0.4 mmol) was added, and 1 mL of 1,2-dichloroethane was added. The reaction tube was sealed, and the reaction tube was placed in an oil bath at 80° C. for 12 hours to react. Track the reaction with TLC. After the reaction is terminated, add ethyl acetate / water for extraction, dry the organic phase with anhydrous sodium sulfate, concentrate the organic phase in vacuo, and then obtain the target compound 3ab by column chromatography with a yield of 84% and an ee value of 98%. . The preparation of products 3aa-3ib refers to the synthesis and post-processing methods of compound 3ab.
Embodiment 3
[0041] In a 10mL vacuum tube, add 5-I-N 3-Bz protected thymine (68.4mg, 0.2mmol), [Rh(COD)Cl 2 ] (2.9 mg, 0.006 mmol) and (R)-DTBM-Segphos (14.2 mg, 0.012 mmol). The reaction tube was filled with argon by replacing it with argon three times, and then, under nitrogen flow, carbonate 2b (82.4 mg, 0.4 mmol) was added, and 1 mL of 1,2-dichloroethane was added. The reaction tube was sealed, and the reaction tube was placed in an oil bath at 80° C. for 12 hours to react. Track the reaction with TLC. After the reaction is terminated, add ethyl acetate / water for extraction, dry the organic phase with anhydrous sodium sulfate, concentrate the organic phase in vacuo, and then obtain the target compound 3cb through column chromatography with a yield of 83% and an ee value of 95%. .
[0042] Representative compound characterization data are as follows:
[0043] 3cb Light yellow oil.[α] D 27 =-115.4° (c=0.92, CH 2 Cl 2 ).HPLC CHIRALCEL IA, n-hexane / 2-propanol=70 / 30, flow rate=0.8mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com