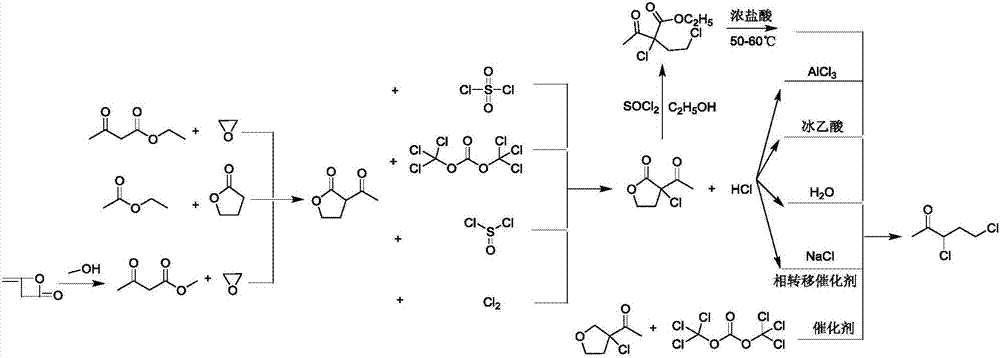
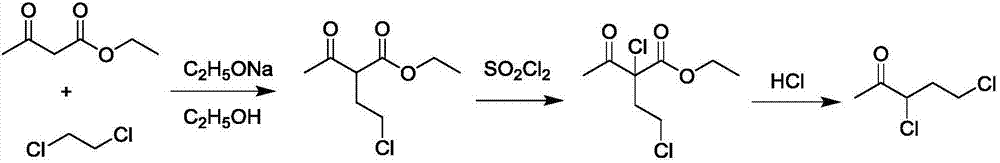
Synthetic method for preparing 3,5-dichloro-2-pentanone with ethyl acetoacetate
A technology of ethyl acetoacetate and a synthesis method, which is applied to the preparation of carboxylate, the preparation of carbon-based compounds, chemical instruments and methods, etc., can solve problems such as hidden safety hazards, complicated handling, flammability and explosion, and prevent reaction coking. , improve the reaction yield, reduce the effect of safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] Above-mentioned synthetic method specifically comprises the following steps:
[0027] (1) Chlorination reaction of 1,2-dichloroethane: Add ethanol solution of sodium ethoxide and 1,2-dichloroethane to the reactor Ⅰ, lower the temperature, then add ethyl acetoacetate dropwise to control the rate of addition, Control the reaction temperature and monitor the reaction degree by gas chromatography. After the reaction is complete, concentrate under reduced pressure, wash with water and separate the liquid, and put the lower organic phase directly into the reactor II;
[0028] (2) Sulfonyl chloride chlorination reaction: cool down the reactor II, add sulfuryl chloride dropwise, the reaction temperature is controlled at 5-10°C, and the reaction degree is monitored by gas chromatography;
[0029] (3) Dilute acid hydrolysis decarboxylation reaction, atmospheric distillation: After the reaction is complete, add water, dilute hydrochloric acid, and tetraethylammonium chloride to re...
Embodiment 1
[0036] A kind of synthetic method of preparing 3,5-dichloro-2-pentanone by ethyl acetoacetate, specifically comprises the following steps:
[0037] Add 245g (3.6mol) of sodium ethylate and 1550mL of ethanol to the reactor I in sequence, stir and dissolve, add 445g (4.5mol) of 1,2-dichloroethane, cool down to 0±1°C, and then start adding acetyl Ethyl acetate 387g (3mol), the rate of addition is controlled, and the reaction temperature is kept between 0-5°C. Use gas chromatography to monitor the reaction degree. After the reaction is complete, concentrate under reduced pressure at 30-40°C; add 500g of water to the concentrated residue and stir for 30min, separate the liquid, and pour the water phase into the waste liquid bucket; add 200g of water to the organic phase and stir for 30min. Separate the liquid, and put the lower organic phase directly into the reactor II. Reactor II was cooled to 5±1°C, 445g (3.3mol) of sulfuryl chloride was added dropwise, the reaction temperature...
Embodiment 2
[0039] A kind of synthetic method of preparing 3,5-dichloro-2-pentanone by ethyl acetoacetate, specifically comprises the following steps:
[0040] Add 204g (3mol) of sodium ethylate and 1291mL of ethanol to the reactor I in sequence, stir and dissolve, add 445g (4.5mol) of 1,2-dichloroethane, cool down to 0±1°C, and then start adding ethyl acetoacetate dropwise 387g (3mol), the rate of addition is controlled, and the reaction temperature is kept between 0-5°C. Use gas chromatography to monitor the reaction degree. After the reaction is complete, concentrate under reduced pressure at 30-40°C; add 500g of water to the concentrated residue and stir for 30min, separate the liquids, and pour the water phase into the waste liquid bucket; add 200g of water to the organic phase and stir for 30min. Separate the liquid, and put the lower organic phase directly into the reactor II. Reactor II was cooled to 5±1°C, 445g (3.3mol) of sulfuryl chloride was added dropwise, the reaction tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com