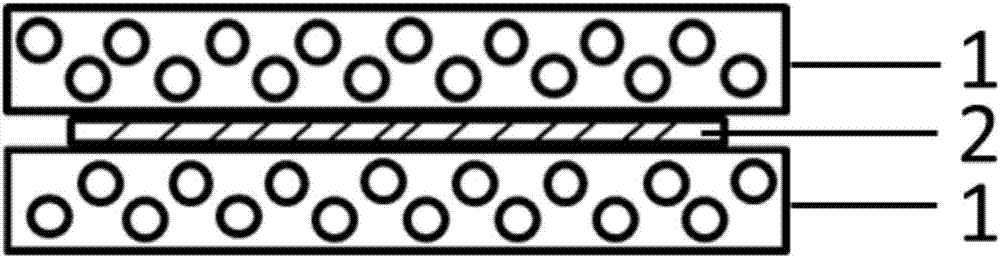
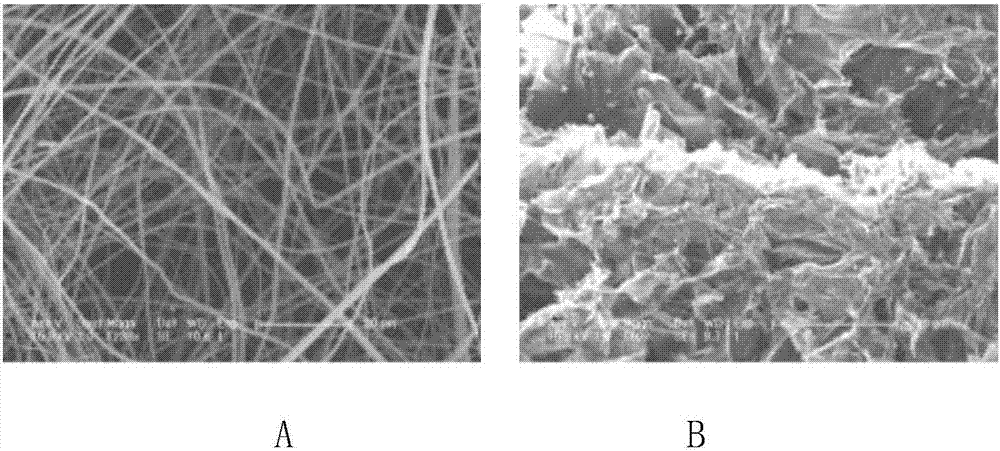
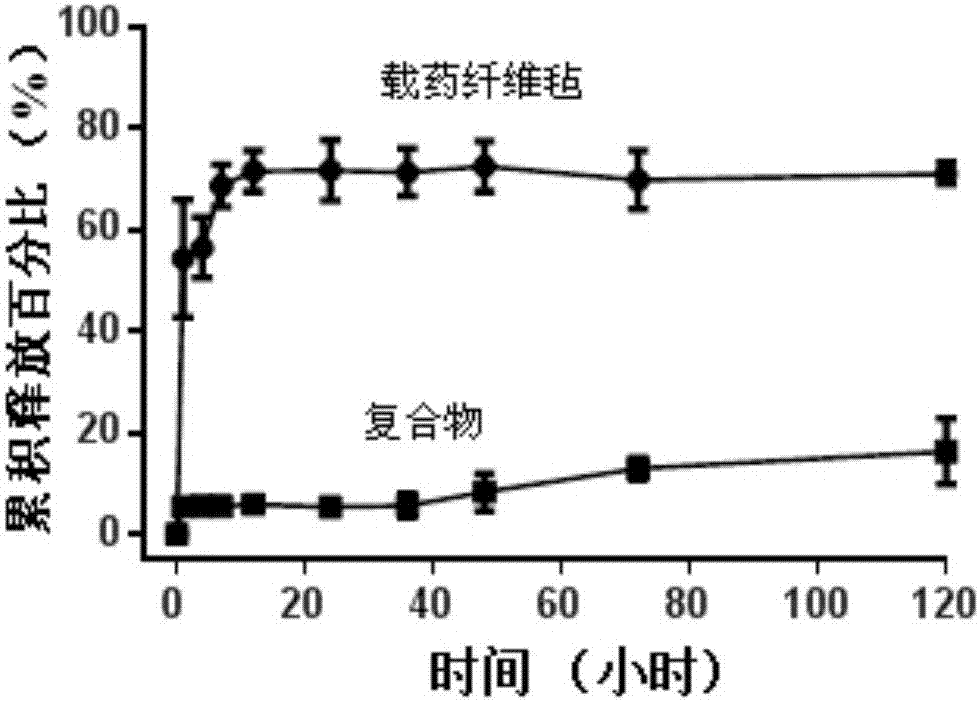
Hemostatic sponge/drug-loaded fiber mat/hemostatic sponge compound and preparation method thereof
A technology of hemostatic sponge and fiber felt, applied in the field of hemostatic sponge/medicine-loaded fiber felt/hemostatic sponge composite and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The present invention also provides a preparation method of a hemostatic sponge / medicated fiber felt / hemostatic sponge composite, the method comprising:
[0028] Step 1: preparing a mixed solution of polymer carrier and antitumor drug, and preparing the mixed solution by electrospinning to obtain a drug-loaded fiber mat;
[0029] Step 2: dissolving the hemostatic sponge material in a solvent to obtain a hemostatic sponge solution, adding a cross-linking agent to the hemostatic sponge solution to obtain a hemostatic sponge precursor solution;
[0030] Step 3: Place the hemostatic sponge precursor solution obtained in step 2 in the mold, and after placing it for 30-40 minutes, place the drug-loaded fiber mat obtained in step 1 on the hemostatic sponge precursor solution, then add the hemostatic sponge precursor solution, and let it stand for a while. After 12-14 hours, the product is freeze-dried to obtain a hemostatic sponge / drug-loaded fiber mat / hemostatic sponge compos...
Embodiment 1
[0043] Dissolve 320mg of PLGA in 3ml of trifluoroethanol, add 80mg of gelatin to 1ml of trifluoroethanol, stir in a water bath at 50°C until completely dissolved, disperse 21.53mg of cisplatin in 500μl of trifluoroethanol, and slowly add it dropwise to the PLGA solution, Stir until uniformly mixed to obtain a mixed solution of PLGA and cisplatin, mix the gelatin solution with the mixed solution of PLGA and cisplatin, stir at room temperature for 2 hours, and use the resulting mixed solution for spinning.
[0044] The resulting spinning solution is immediately spun, and the electrospinning operation parameters involved are as follows: the diameter of the spinneret is 0.4mm, the strength of the applied electrostatic field is 30KV, and the distance between the spinneret and the receiving screen The flow rate of the solution was 1ml / h, and the spinning was all carried out at room temperature to obtain a drug-loaded fiber mat, which was vacuum-dried at 25°C.
[0045] Chitosan was d...
Embodiment 2
[0053] Dissolve 240mg of PCL in 3ml of trifluoroacetic acid, add 80mg of collagen into 1ml of trifluoroacetic acid, stir until completely dissolved, mix the PCL solution and collagen solution evenly, dissolve 16mg of paclitaxel in 500μl of trifluoroacetic acid, then slowly It was added dropwise to the mixed solution of PCL and collagen, stirred at room temperature for 2.5 hours, and the obtained mixed solution was used for spinning.
[0054] The obtained spinning solution is immediately spun, and the electrospinning operation parameters involved are as follows: the diameter of the spinneret is 0.5mm, the strength of the applied electrostatic field is 20KV, the distance between the spinneret and the receiving screen The flow rate of the solution was 0.8ml / h, and the spinning was all carried out at room temperature to obtain a drug-loaded fiber mat, which was vacuum-dried at 25°C.
[0055] Dissolving chitosan in 0.7% acetic acid solution to obtain a chitosan solution with a conc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Aperture diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com