Antibody for resisting human epidermal growth factor receptor 2 (HER2), as well as medical composition and use of antibody
An antibody and antigen technology, applied in the field of tumor therapy and molecular immunology, can solve the problems of drug resistance, abnormal activation of receptor signaling pathways, poor efficacy of breast cancer, etc., and achieve the effect of inhibiting tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Embodiment 1: Amino acid sequence design and gene sequence optimization of the heavy chain and light chain of antibodies MIL203 and MIL204 change
[0158] (1) Amino acid sequence of MIL203 light chain and heavy chain
[0159] The heavy chain amino acid sequence of MIL203 is as follows, wherein the underlined part is the amino acid sequence of the heavy chain variable region:
[0160] EVQLVESGGGLVQPGGSLRLSCAASGFNIKDTYIHWVRQAPGKGLEWVARIYPTNGYTRYADSVKGRFTISADTSKN TAYLQMNSLRAEDTAVYYCSRWGGDGFYAMDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLSCAVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLVSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK(SEQ ID NO:22)
[0161] The light chain amino acid sequence of MIL203 is as follows, wherein the underlined part is the amino acid sequenc...
Embodiment 2
[0179] Embodiment 2: Construction of 203 antibody eukaryotic expression vector and 204 antibody eukaryotic expression vector
[0180] Using the expression vector pTGS-FRT-DHFR (please refer to the Chinese authorized invention patent ZL200510064335.0 for the construction method), remove the hygromycin selection tag, and add GS (glutamine synthetase, glutamine synthetase) expression through the PshA1 and Xho1 restriction sites box, as a selection marker; where GS cDNA can be obtained by RT-PCR from the cell line CHO expressing GS. The transformed vector was named GS vector.
[0181] On the basis of the GS vector, insert the fully synthetic light chain constant region (the constant region sequence is the non-underlined sequence in SEQ ID NO:24 or SEQ ID NO:27) through the restriction sites of BsiwI and NotI; after that, pass The restriction sites of Nhe I and XhoI were respectively inserted into the fully synthesized 203 heavy chain constant region and 204 heavy chain constant...
Embodiment 3
[0187] Example 3: Host cell fucose knockout and suspension acclimatization
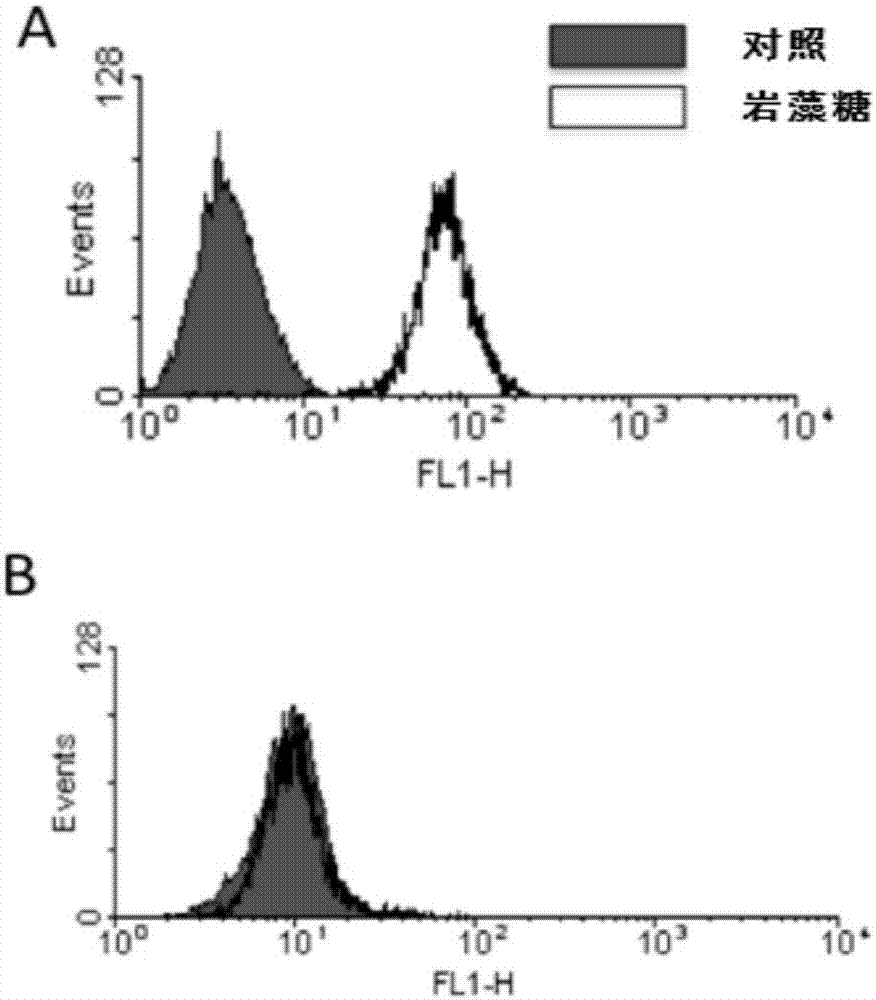
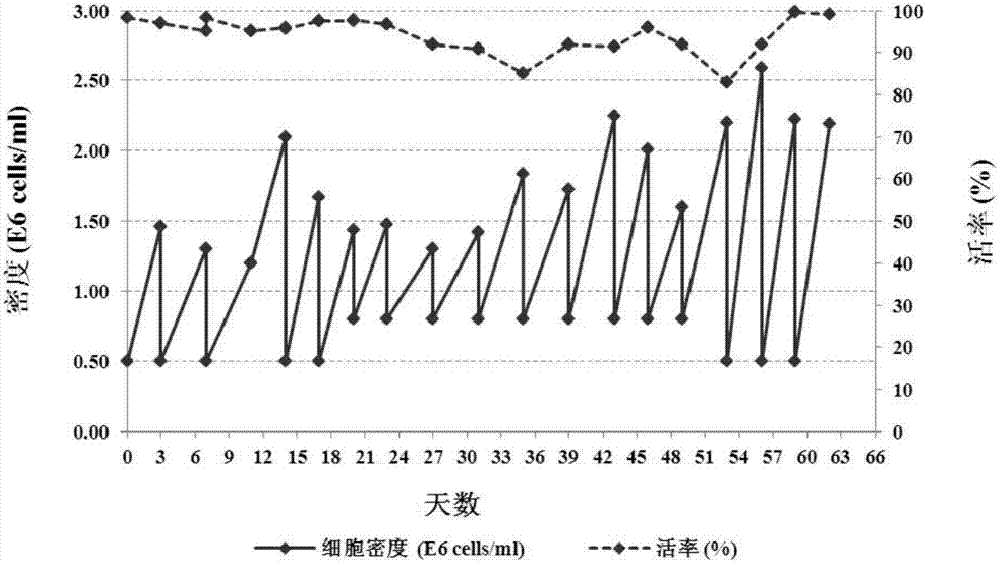
[0188] The CHO-K1 cells purchased from ATCC (ATCC: 58995535) were gene knocked out so that the proteins expressed by themselves had almost or no fucosylation modification, and the obtained fucose knockout host cells were named CHOK1-AF, The specific method is to transform the expression system through genetic engineering technology, and knock out the key protein GFT of the fucose modification pathway in the antibody expression host cell CHO-K1, thereby effectively reducing the level of antibody fucose modification. The method can simultaneously block the classical fucosylation pathway and the compensation pathway, thereby achieving the purpose of completely removing fucosylation. Specific technical routes such as figure 1As shown, using the zinc finger enzyme technology, two GFT zinc-finger nuclease zinc finger enzyme sequences were designed for the sequence of the GFT gene SLC35c1 (GenBank: BAE16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com