Rectifying separation method for chlorinated benzene production
A rectification separation and chlorinated benzene technology, applied in chemical instruments and methods, halogenated hydrocarbon disproportionation separation/purification, organic chemistry, etc., can solve the problem of increasing the separation cost of chlorinated benzene, increasing the cost of environmental treatment, and the crystallization effect is not very good Ideal and other issues, to achieve the effect of increasing the rate and reaction progress, saving synthesis costs, and meeting the requirements of process design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
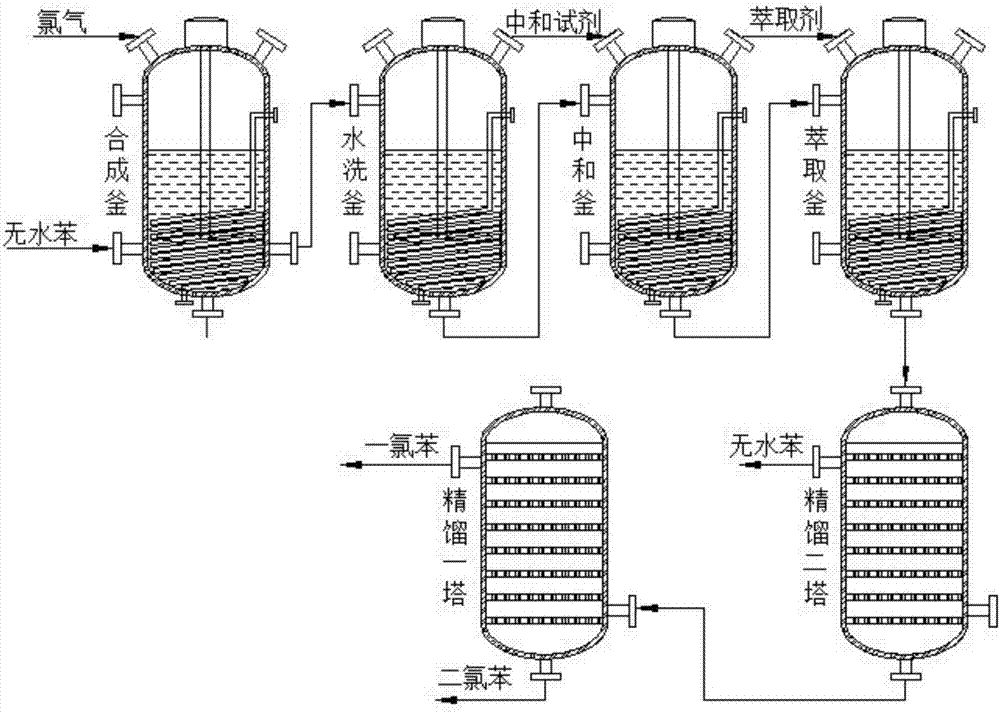
[0026] see figure 1 As shown, the technical scheme adopted in the present invention is: a rectification and separation method for the production of chlorinated benzene, which specifically includes the following steps:
[0027] (1). Synthesis: Pass chlorine gas and excess anhydrous benzene into a chlorinator equipped with an active catalyst, set the reaction temperature and stirring rate inside the chlorinator, and turn on the agitator to react to generate chlorinated benzene Chlorinated solution;
[0028] (2). Washing: transfer the chlorinated liquid containing chlorinated benzene obtained from the chlorinator into the washing tank, set the temperature of the water in the washing tank and the stirring speed in the washing tank, and wash with water. The chlorine after washing The chemical solution is transferred to the neutralization tank;
[0029] (3). Neutralization: transfer to the chlorinated solution in the neutralization tank and add neutralization reagent, set the stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com