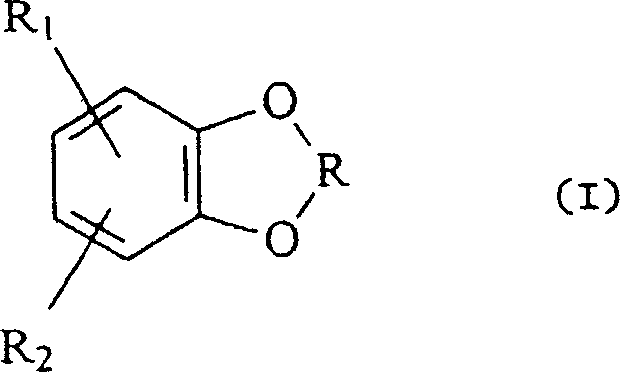
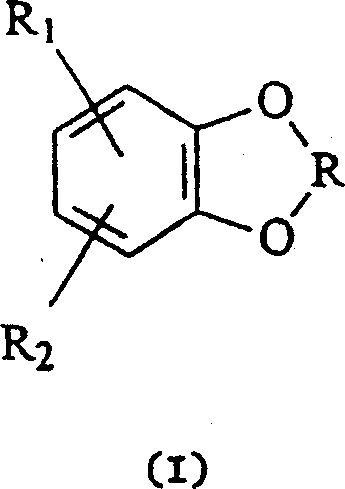
Hydroxylation process for aromatic compounds containing a dioxa-heterocyclic system
A technology for the hydroxylation of aromatic compounds, applied in the field of hydroxylation of aromatic compounds, can solve problems such as difficult to operate reagents, expensive raw materials, and environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 30 g of TS-1 catalyst, 150 g of methyl isobutyl ketone, 150 g of acetone and 150 g (1.23 moles) of MDB were charged into a reactor equipped with a stirrer, thermometer, condenser and heating / cooling jacket. The resulting suspension was heated to 60°C, maintaining the mass under agitation. Add 84 g of 10% (0.25 mol) H in about 4 hours 2 o 2 , the temperature of the suspension was maintained at 60 °C. The suspension was stirred at 60° C. for 1 hour and the organic phase was subsequently analyzed after separation from the TS-1 catalyst.
[0033] The following results were obtained from GLC analysis of the organic phase: MDB conversion = 15%; selectivity to 5-hydroxy-MDB = 62%.
Embodiment 2
[0035] The same procedure as Example 1 was used, but only 300 g of acetone was added instead of the acetone / methyl isobutyl ketone mixture.
[0036] The following results were obtained from GLC analysis of the organic phase: MDB conversion = 12%; selectivity to 5-hydroxy-MDB = 58%.
Embodiment 3
[0038] Using the same method as Example 1, but dosing 70 g of H in place of the solvent mixture 2 O and 28 g of 30% (0.25 mole) H 2 o 2 .
[0039] The following results were obtained from GLC analysis of the organic phase: MDB conversion = 10%; selectivity to 5-hydroxy-MDB = 25%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com