A kind of method utilizing cyclohexene and acetic acid to prepare cyclohexanol and cyclohexyl acetate
A technology of cyclohexyl acetate and cyclohexene, which is applied to the preparation of carboxylic acid esters, chemical instruments and methods, hydrolysis preparation, etc., can solve the problems of high price, low selectivity, and large hydrogen consumption, and achieve low production costs , high selectivity, flexible control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
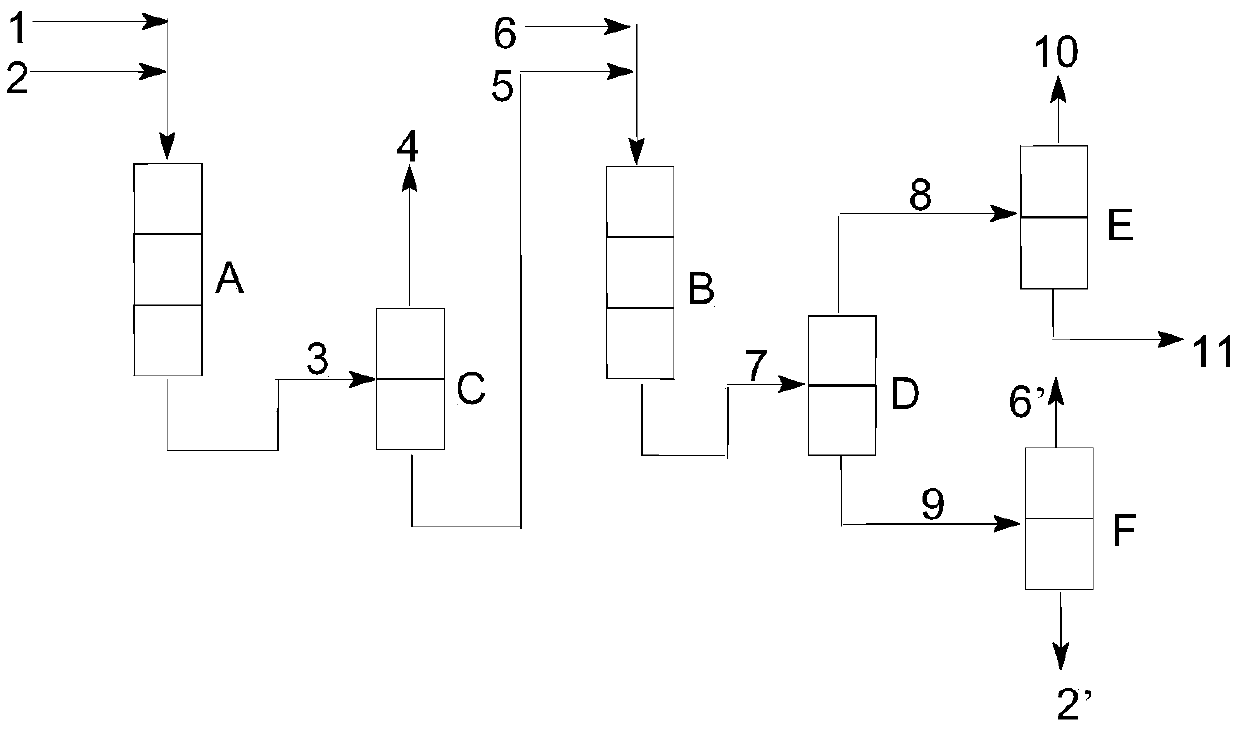
[0046] Reaction raw materials: cyclohexene is an industrial product with a purity of more than 99.5%, acetic acid is glacial acetic acid with a purity of more than 99.5%, and water is deionized water. Both the first catalyst and the second catalyst are H-type macroporous strong acid styrene series cations Resin, brand NKC-9, 50 g of the first catalyst was charged into the first reactor A, and 50 g of the second catalyst was charged into the second reactor B.
[0047] Step i: The cyclohexene 1 and the acetic acid 2 are respectively charged from the top of the first reactor A by a metering pump and then reacted under the action of the first catalyst to obtain a mixture 3. The reaction conditions were as follows: the catalyst bed temperature was 90 °C; the catalyst bed height-diameter ratio was 10; the reaction pressure was 1.5 MPa; the molar ratio of cyclohexene to acetic acid was 0.8; the weight space velocity of cyclohexene was 2 h -1 .
[0048] The mixture 3 flows into the f...
Embodiment 2
[0055] Reaction raw materials: the same as Example 1, the difference is: the first catalyst and the second catalyst both adopt modified H-type macroporous strong acid styrene series cationic resin NKC-9, and 50g of the first catalyst is loaded into the first reaction In vessel A, 50 g of the second catalyst was charged into second reactor B.
[0056] The preparation method of modified H-type macroporous strong acid styrene series cationic resin NKC-9:
[0057] 2.0 g of sulfamic acid and 200 mL of methanol were respectively added to a 500 mL round-bottomed flask, and stirred to dissolve the sulfamic acid. 180g of NKC-9 resin and the methanol solution dissolved with sulfamic acid were added to the autoclave respectively, the autoclave was installed, heated to 80°C under stirring, cooled after 2 hours, stopped stirring, and the temperature of the autoclave decreased to 80°C. After room temperature, the reaction kettle was disassembled, the catalyst was poured out, dried in the a...
Embodiment 3
[0063] Reaction raw materials: the same as in Example 1.
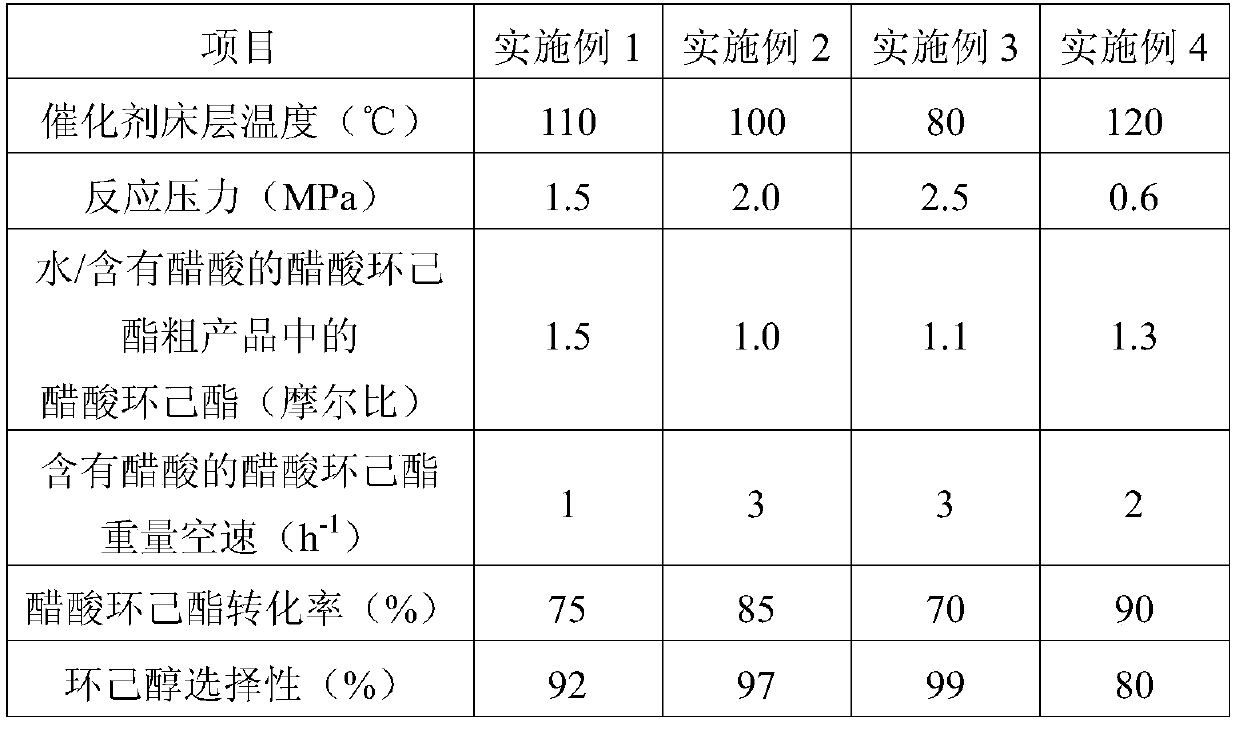
[0064] Step i: The operation is the same as that in Example 1, except that: the catalyst bed temperature is 95° C.; the reaction pressure is 1.0 MPa; and the molar ratio of cyclohexene to acetic acid is 0.70.
[0065] Step ii: the operation is the same as that in Example 1, except that the catalyst bed temperature is 80°C; the reaction pressure is 2.5MPa; the molar ratio of water and the cyclohexyl acetate in the crude cyclohexyl acetate product containing acetic acid is 1.1; The weight space velocity of the cyclohexyl acetate crude product containing acetic acid is 3h -1 .
[0066] The operations of step iii and step iv are the same as in Example 1.
[0067] The reaction conditions and reaction results of step i are shown in Table 1; the reaction conditions and reaction results of step ii are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com