Method for synthesizing zinc-doped copper-indium-sulfur (Zn-doped CuInS2) quantum dots
A synthesis method and technology of quantum dots, applied in chemical instruments and methods, nanotechnology for materials and surface science, electrolytic capacitors, etc. problems such as poor performance, to achieve the effects of composite suppression of internal defects, stable structure, and simple and controllable methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] Preparation of indium source
[0046] Take by weighing 5.13g (30mmol) sodium diethyldithiocarbamate (Nadedc) and add in the beaker, add the deionized water of 200mL in the beaker, stir at room temperature for 30 minutes and make it dissolve completely (the aqueous solution is colorless, clear and transparent) ) to obtain sodium diethyldithiocarbamate aqueous solution; claim 2.92g (10mmol) indium acetate and join in another beaker, add 50mL deionized water in this beaker, stir at room temperature for 15 minutes and make it dissolve completely (the aqueous solution is Colorless, clear and transparent) to obtain indium acetate aqueous solution; the prepared indium acetate aqueous solution is dripped into the aqueous solution of sodium diethyldithiocarbamate (beginning to turn white in the solution) at 1 drop / second (with a 5mL plastic straw). Turbidity), after the dropwise addition, continue magnetic stirring at room temperature for 3 hours to completely react the raw mate...
Embodiment 1
[0047] Example 1: Zn-doped CuInS 2 Preparation of quantum dots
[0048] Weigh 18mg (0.05mmol) copper diethyldithiocarbamate, 55.7mg (0.1mmol) indium diethyldithiocarbamate, 1.8mg (0.005mmol) zinc diethyldithiocarbamate, 3ml Oleylamine was placed in a 50ml round-bottomed flask, placed in a constant temperature oil bath at 180°C for 20 minutes, and then cooled to room temperature. Centrifuge the solution after the reaction, discard the precipitate, wash the supernatant with n-hexane and centrifuge, discard the precipitate again, add ethanol to the supernatant and centrifuge, repeat the washing and centrifugation several times until the centrifuged The supernatant was colorless and transparent, and the final precipitate was taken as the final product.
[0049] Product composition, structure and morphology characterization:
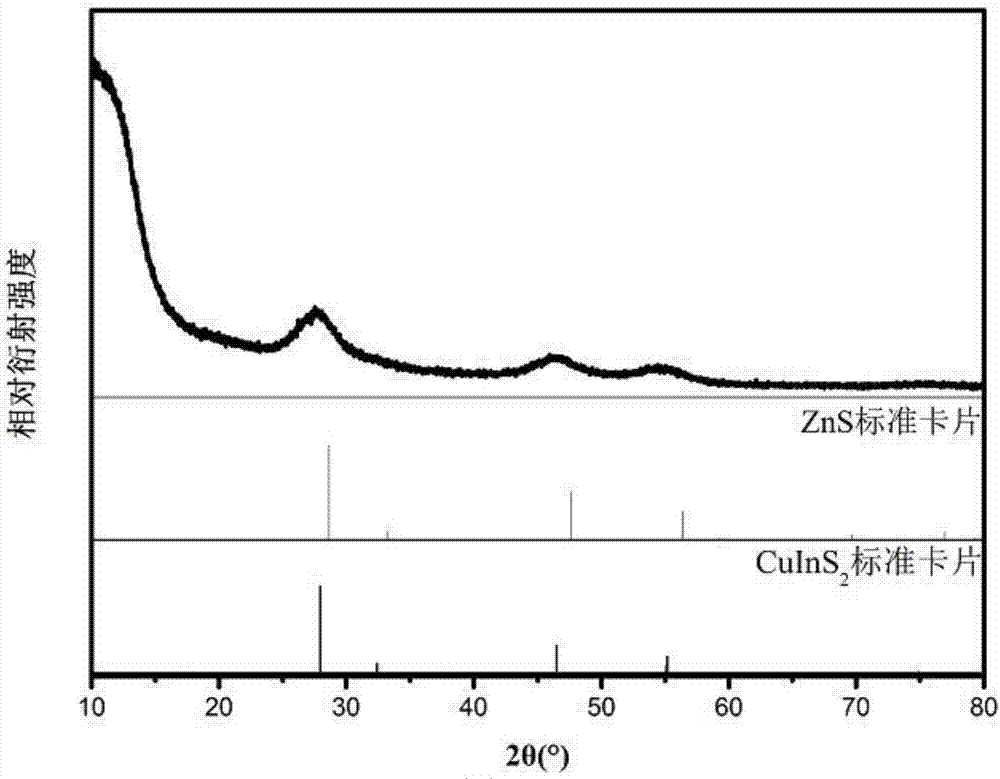
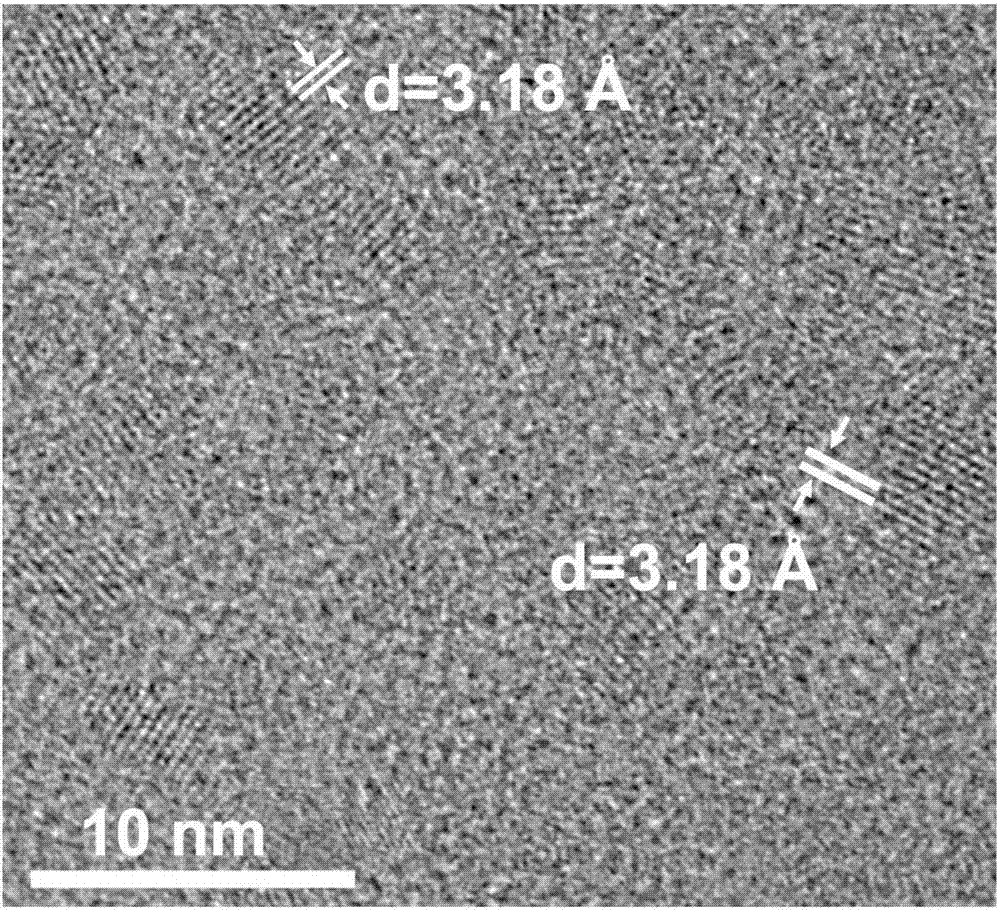
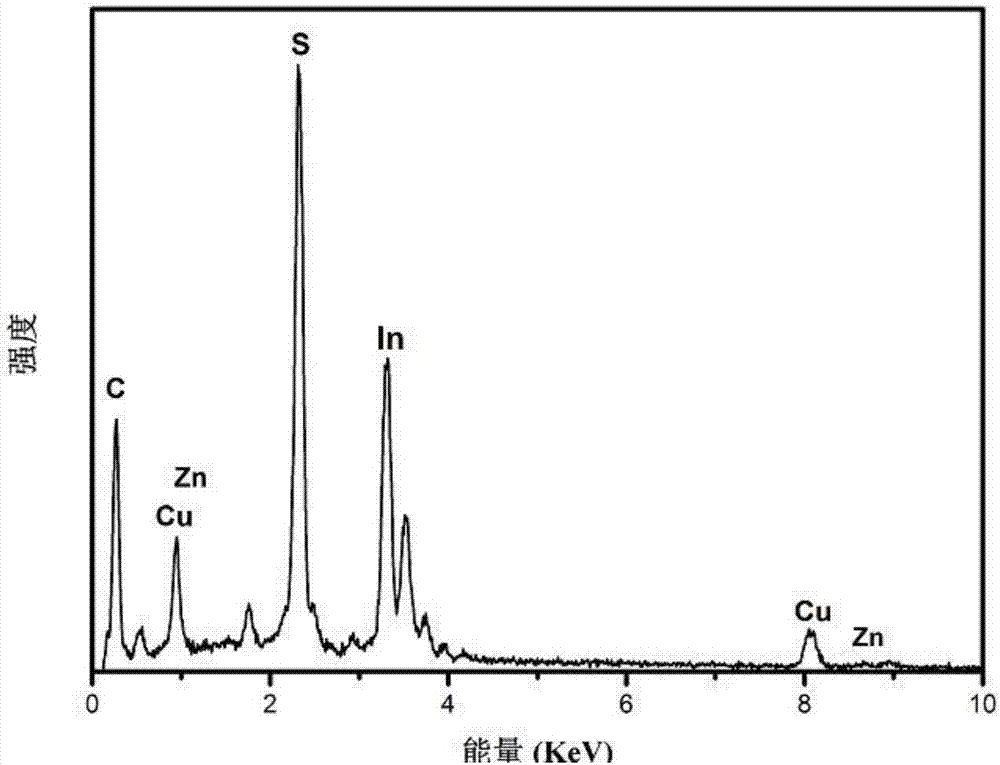
[0050] The final product is dissolved in dichloromethane, and after the dichloromethane volatilizes, the product is subjected to XRD measurement, and the ...
Embodiment 2
[0080] Weigh 18mg (0.05mmol) copper diethyldithiocarbamate, 55.7mg (0.1mmol) indium diethyldithiocarbamate, 18.1mg (0.05mmol) zinc diethyldithiocarbamate, 3ml Oleylamine was placed in a 50ml round-bottomed flask, placed in a constant temperature oil bath at 180°C for 20 minutes, and then cooled to room temperature. Centrifuge the solution after the reaction, discard the precipitate, wash the supernatant with n-hexane and centrifuge, discard the precipitate again, add ethanol to the supernatant and centrifuge, repeat the washing and centrifugation several times until the centrifuged The supernatant was colorless and transparent, and the final precipitate was taken as the final product.
[0081] After identification, the same as Example 1, the final product of Example 2 is Zn-doped CuInS 2 Quantum dots with a size of 4-5nm and good dispersion.
[0082] Example 2 is prepared to obtain the product and carry out the result of XRD test such as Figure 7 shown. From Figure 7 It...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current density | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com