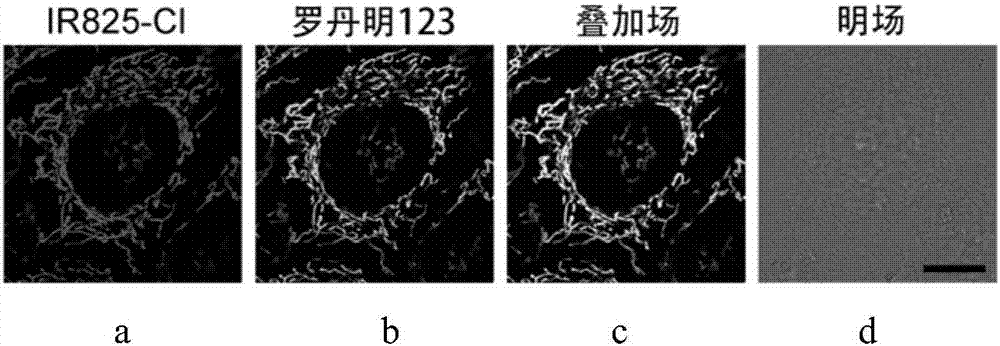
Application of heptamethine indocyanine organic dye as mitochondria fluorescent probe
A technology of heptamethine indocyanines and organic dyes, which is applied in the direction of organic dyes, methine/polymethine dyes, fluorescence/phosphorescence, etc., which can solve the disadvantages of near-infrared light observation, low fluorescence quantum yield, and lack of observation Near-infrared fluorescence and other problems, to achieve excellent targeting effect, high fluorescence quantum yield, and improve the effect of imaging quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthetic process of the mitochondrial fluorescent probe (hereinafter referred to as IR825-Cl) is as follows:
[0030]
[0031] Step 1: Using toluene as a solvent, 2,3,3-trimethyl-4,5-benzo-3H-indole reacted with benzyl bromide in the dark under the protection of nitrogen, and refluxed for 12 hours; where 2,3, The molar ratio of 3-trimethyl-4,5-benzo-3H-indole to benzyl bromide is 1:1, and the resulting reaction product is recrystallized from isopropanol to obtain compound 1;
[0032] Step 2: dichloromethane as solvent, N,N-dimethylformamide (DMF) as reagent and solvent, cyclohexanone and POCl 3 Vilsmeimer-Haack formylation reaction takes place, reacts for 4 hours; Wherein cyclohexanone: POCl 3 : The molar ratio of DMF is 1:2.3:5.4; The obtained product obtains compound 2 through ethyl acetate recrystallization;
[0033] Step 3: The above two products 1 and 2 obtained above are co-dissolved in acetic anhydride with anhydrous sodium acetate, and reacted under ni...
Embodiment 2
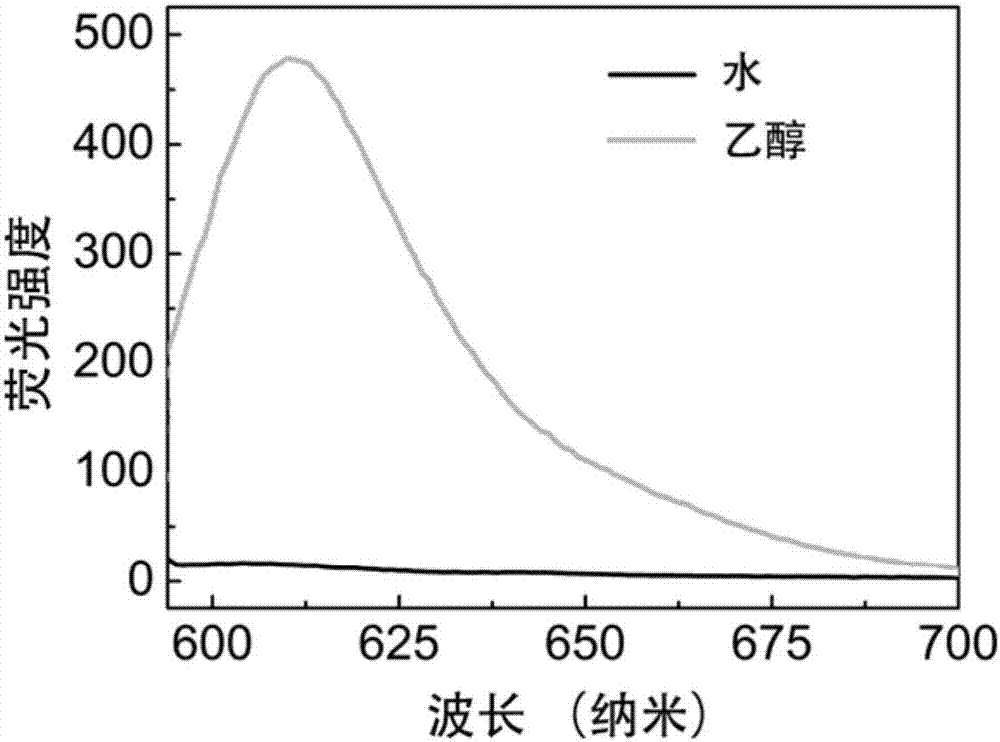
[0035] Measure the fluorescence spectrum of the IR825-Cl that embodiment 1 makes in water and ethanol, and its method is as follows:
[0036] Dissolve IR825-Cl in dimethyl sulfoxide to prepare a 50mg / mL stock solution, and then dilute the stock solution with water and ethanol to a 10μg / mL working solution. The fluorescence properties of the two solutions were characterized by fluorescence spectrometer. see results figure 1 .
[0037] As shown in the figure, the IR825-Cl prepared in Example 1 has almost no fluorescence in water, but emits strong red fluorescence at around 610 nm in ethanol.
Embodiment 3
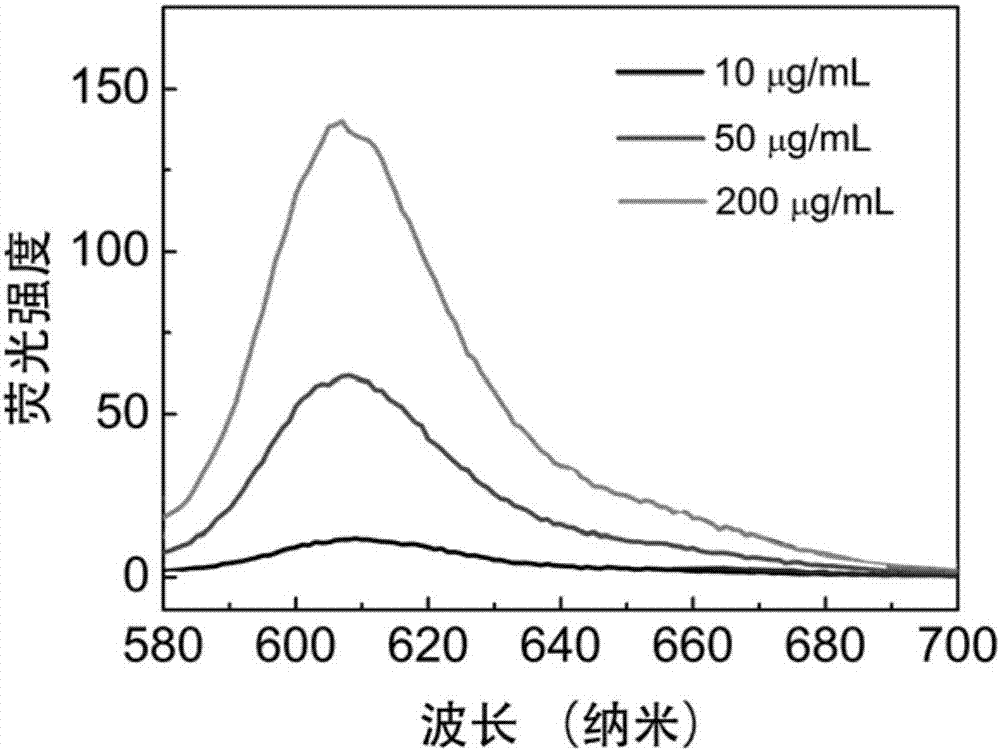
[0039] Measure the fluorescence spectrum of the IR825-Cl that embodiment 1 makes in the liposome solution of different concentrations, its method is as follows:
[0040] Dissolve IR825-Cl in dimethyl sulfoxide to prepare a 50 mg / mL stock solution, and then dilute the stock solution with phosphate buffered solution (PBS) to a 20 μg / mL working solution. Weigh 4 mg of palmitoyloleoylphosphatidylcholine (POPC), add 1 mL of PBS for hydration, and then prepare a liposome solution of POPC by ultrasonic method. The liposome solution was diluted with PBS to solutions with concentrations of 20, 100 and 400 μg / mL, respectively, and then mixed with the above-mentioned IR825-Cl working solution in equal volume to prepare the final sample. The fluorescence properties of these three samples were characterized by fluorescence spectrometer. see results figure 2 .
[0041] As shown in the figure, as the concentration of the liposome solution increases, the red fluorescence of the IR825-Cl p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com