Water-soluble copper (II) complex as well as synthesis method and application thereof
A synthesis method and technology of complexes, applied in the field of medicine, can solve the problems of high research and development cost, existence of side effects and water solubility, and difficulty in research and development, and achieve the effects of good medicinal value, good water solubility and low liver cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the synthesis of N-(4-benzoic acid-2-diethylaminoethyl)-8-quinoline aldimine hydrochloride ligand (hereinafter referred to as ligand)
[0037] Add 1.57g of quinoline-8-carbaldehyde and 2.71g of procaine hydrochloride into a 250mL round bottom flask, then add 50ml of dichloromethane, 2.1ml of tetraethyl titanate, stir at room temperature for 24 hours, then add 7.1g of anhydrous sulfuric acid Sodium, stirred for 6 hours, filtered with diatomaceous earth, the filtrate was spin-dried, dissolved in a small amount of dichloromethane, recrystallized at -30°C, the solid was separated and dried to obtain a light yellow powder product.
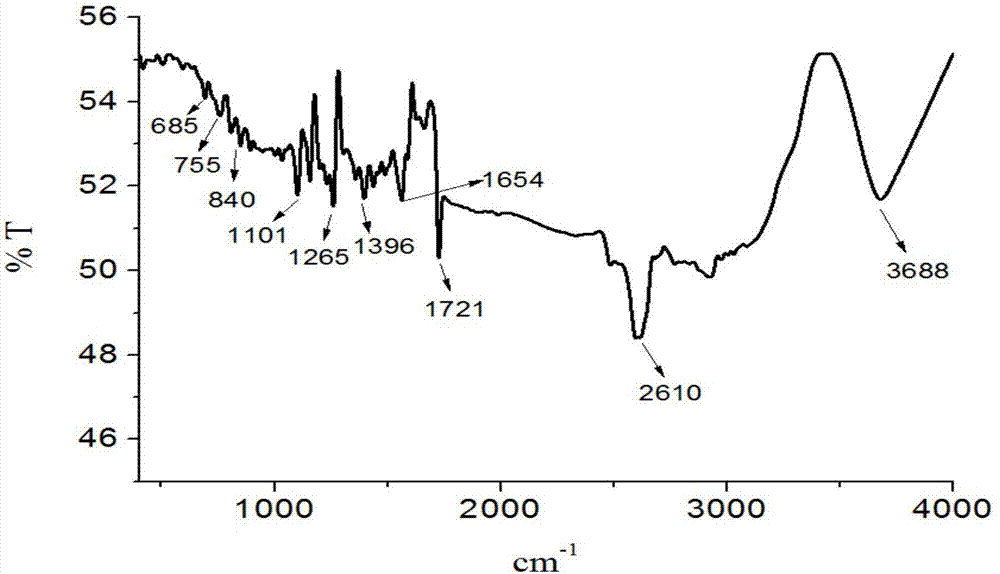
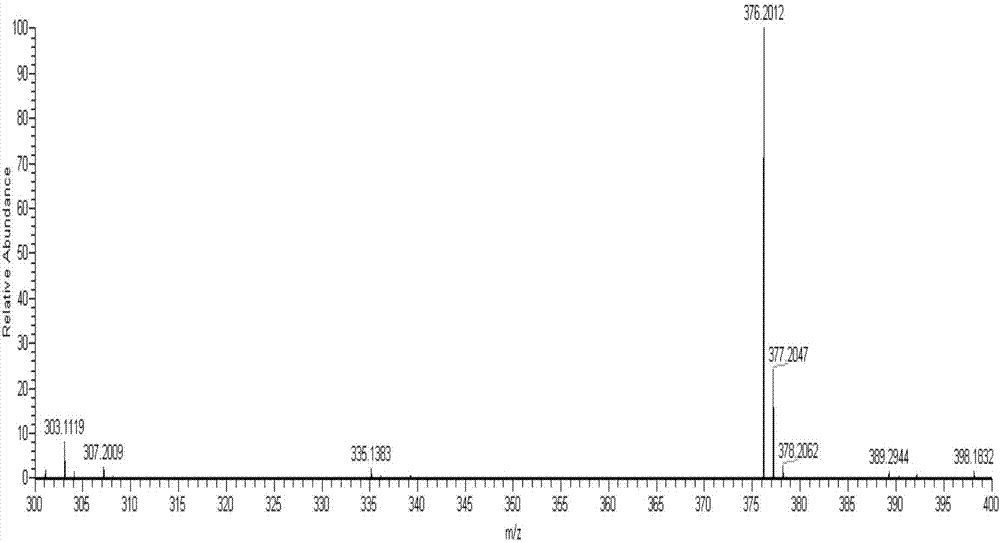
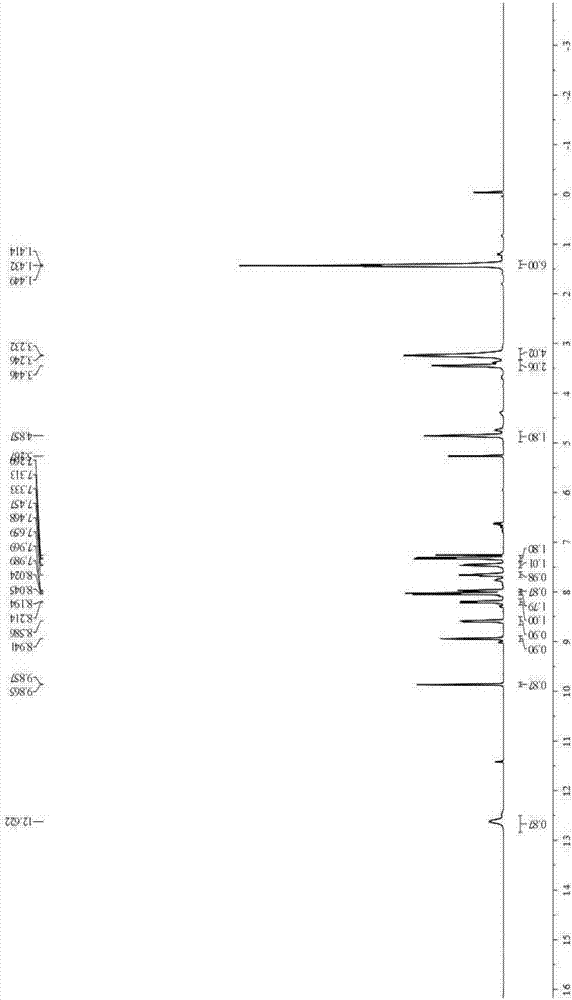
[0038] The product obtained in this embodiment is analyzed by infrared spectrum, high-resolution mass spectrometry and proton nuclear magnetic resonance spectrum, and its collection of spectra is as follows: figure 1 , figure 2 and image 3 As shown, therefore, it can be determined that the resulting product is N-(4-benzoic acid-...
Embodiment 2
[0039] Embodiment 2: the synthesis of ligand
[0040] Repeat Example 1, the difference is: use filter paper suction filtration instead of diatomaceous earth filtration.
[0041] The product obtained in this example is analyzed by infrared spectrum, high-resolution mass spectrometry and proton nuclear magnetic resonance spectrum, and it can be determined that the product obtained is N-(4-benzoic acid-2-diethylaminoethyl)-8-quinoline aldimine Hydrochloride.
Embodiment 3
[0042] Embodiment 3: the synthesis of ligand
[0043] Repeat Example 1, except that no anhydrous sodium sulfate is added after the reaction is complete.
[0044] The product obtained in this example is analyzed by infrared spectrum, high-resolution mass spectrometry and proton nuclear magnetic resonance spectrum, and it can be determined that the product obtained is N-(4-benzoic acid-2-diethylaminoethyl)-8-quinoline aldimine Hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com