Tilmicosin film-controlled enteric-coated sustained release preparation and preparation method thereof
A technology of tilmicosin and sustained-release preparations, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the unfavorable recycling of waste materials and the instability of coated granules , Easy to degrade gastric mucosa and other problems, to achieve the effect of reducing the clinical addition of drugs, improving drug efficacy and bioavailability, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
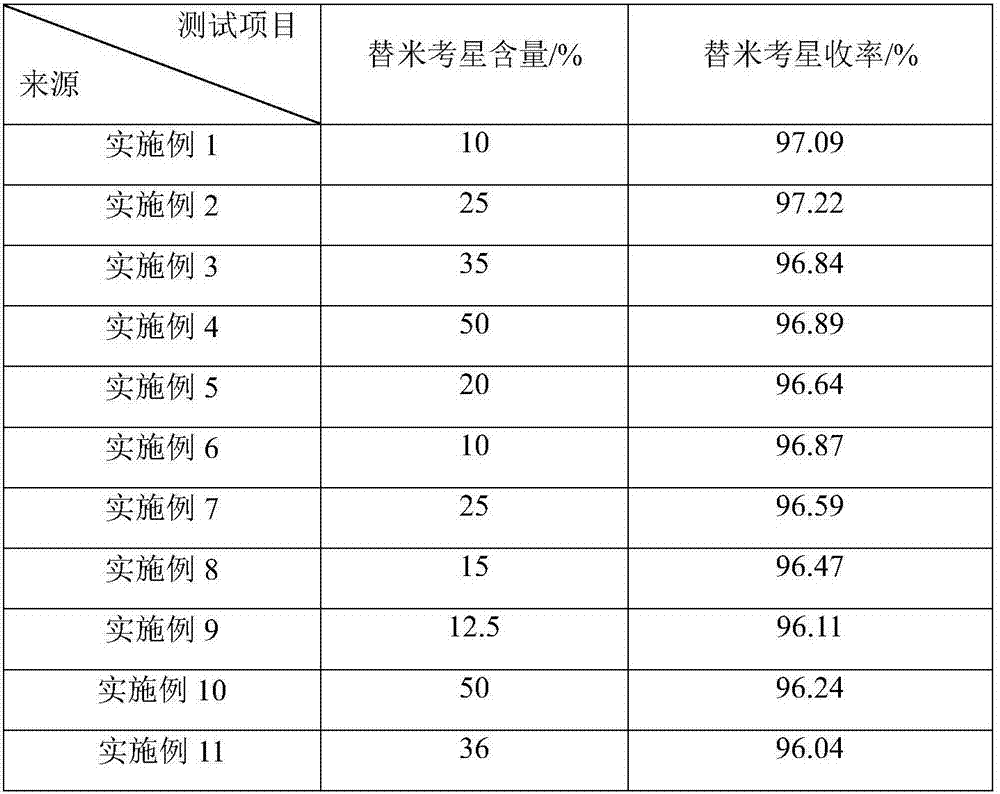
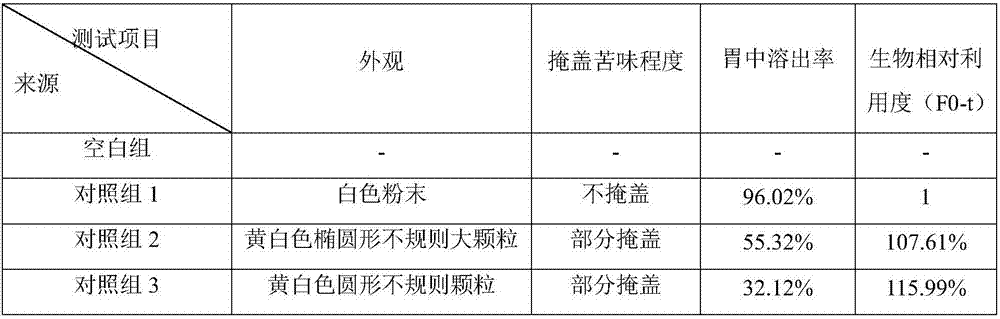
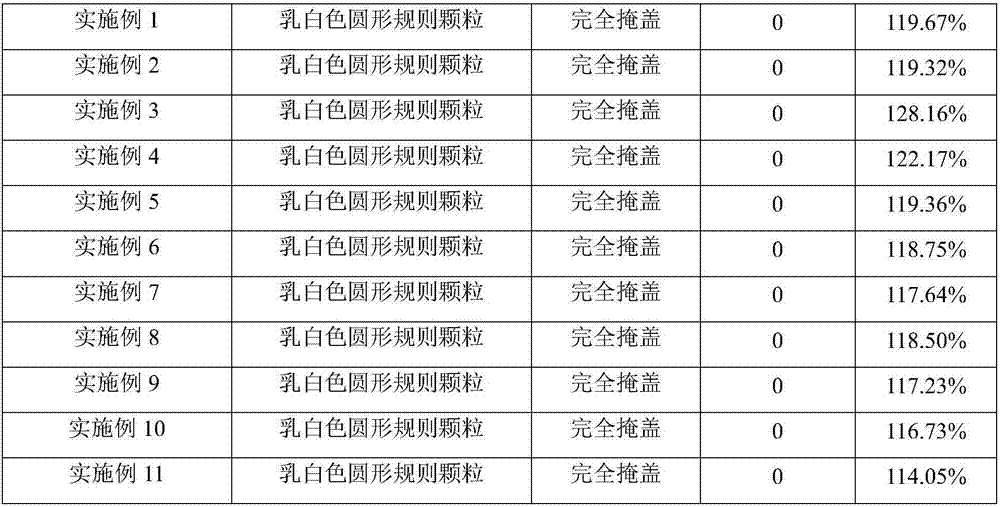
Examples
Embodiment 1
[0036] Tilmicosin film-controlled enteric-coated sustained-release preparation, which consists of:
[0037] Tilmicosin powder 10g;
[0038] Inner layer excipients: starch 57.08g, microcrystalline cellulose 15g, sodium alginate 6.2g, chitin 3.72g, hydroxypropyl methylcellulose 0.12g;
[0039] Film coating layer material: polyvinyl alcohol acetate phthalate 5.72g;
[0040] Excipient materials for film coating layer: 0.16 g of talc powder, 2 g of titanium dioxide.
[0041] The preparation method of tilmicosin film-controlled enteric-coated sustained-release preparation, the specific steps are:
[0042] S1. Mix the original powder of tilmicosin and the excipients of the core layer except hydroxypropyl methylcellulose according to the method of equal increase;
[0043] S2. Dissolving hydroxypropyl methylcellulose in ethanol to prepare an ethanol solution with a mass fraction of 10%;
[0044] S3. Add the mixture obtained in step S1 to the ethanol solution obtained in step S2 to ...
Embodiment 2
[0049] Tilmicosin film-controlled enteric-coated sustained-release preparation, consisting of:
[0050] tilmicosin powder 25g;
[0051] Inner layer excipients: starch 47.08g, microcrystalline cellulose 10g, sodium alginate 6.2g, chitin 3.72g, hydroxypropyl methylcellulose 0.12g
[0052] Film coating layer material: polyvinyl alcohol acetate phthalate 5.72g
[0053] Excipient materials for film coating layer: 0.16 g of talc powder, 2 g of titanium dioxide.
[0054] The preparation method of tilmicosin film-controlled enteric-coated sustained-release preparation, the specific steps are:
[0055] S1. Mix the original powder of tilmicosin and the excipients of the core layer except hydroxypropyl methylcellulose according to the method of equal increase;
[0056] S2. Dissolving hydroxypropyl methylcellulose in ethanol to prepare an ethanol solution with a mass fraction of 10%;
[0057] S3. Add the mixture obtained in step S1 to the ethanol solution obtained in step S2 to make a...
Embodiment 3
[0062] Tilmicosin film-controlled enteric-coated sustained-release preparation, consisting of:
[0063] tilmicosin powder 35g;
[0064] Inner layer excipients: starch 28.84g, povidone 4g, microcrystalline cellulose 15g, sodium alginate 6.2g, chitin 3.72g, hydroxypropyl methylcellulose 0.24g;
[0065] Film coating layer material: polyacrylic acid resin 5g;
[0066] Excipients for the film coating layer: 0.5 g of magnesium stearate, 1.5 g of titanium dioxide.
[0067] The preparation method of tilmicosin film-controlled enteric-coated sustained-release preparation, the specific steps are:
[0068] S1. Mix the original powder of tilmicosin and the excipients of the core layer except hydroxypropyl methylcellulose according to the method of equal increase;
[0069] S2. Dissolving hydroxypropyl methylcellulose in ethanol to prepare an ethanol solution with a mass fraction of 10%;
[0070] S3. Add the mixture obtained in step S1 to the ethanol solution obtained in step S2 to make...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com