Naphthoquinone dimer and preparation method and applications thereof
A naphthoquinone dimer and carrier technology, applied in the field of medicine, can solve problems such as no naphthoquinone dimer, and achieve the effects of good controllability and reproducibility, low cost and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
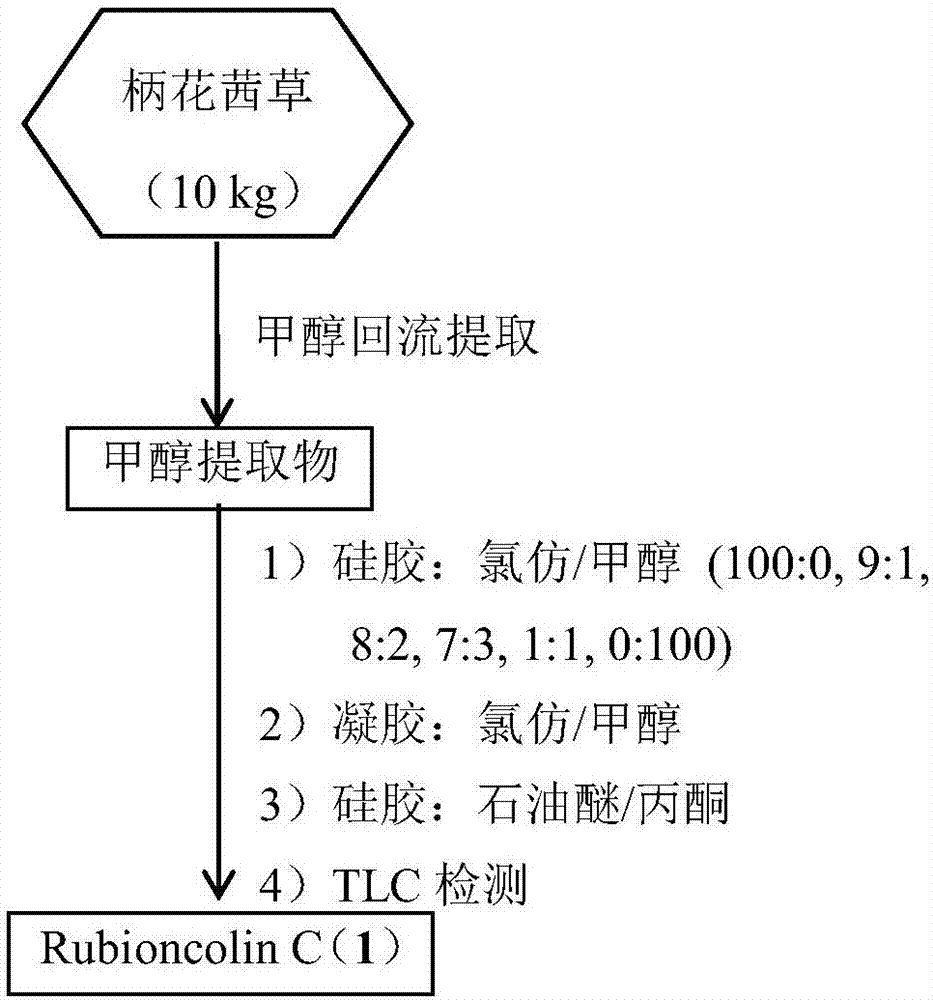
[0030] The preparation method of naphthoquinone dimer Rubioncolin C (1) (flow process sees figure 1 ), including the following steps:
[0031] Take 10kg of roots and rhizomes of Rubia petioli, after drying and crushing, extract 3 times under methanol reflux (20L×3), the time is 4h, 3h and 3h, and the total methanol extract after the extract is concentrated under reduced pressure is 1.42kg. The total extract was chromatographed on a silica gel column and eluted with a gradient of chloroform / methanol (100:0, 9:1, 8:2, 7:3, 1:1, 0:100) to obtain six components Fr.1 -Fr.6; wherein the low polar part Fr.1 (53g) was subjected to silica gel column chromatography and eluted with petroleum ether / acetone (30:1, 15:1, 5:1, 1:1) gradient to obtain 4 subfractions Fr.1-1-Fr.1-4; Fr.1-3 (17g) subfractions were subjected to Sephadex LH-20 gel column chromatography, 1:1 chloroform / methanol as eluent , to obtain 3 subcomponents Fr.1-3-1-Fr.1-3-3; Fr.1-3-2 (5.2g) was repeatedly subjected to si...
Embodiment 2
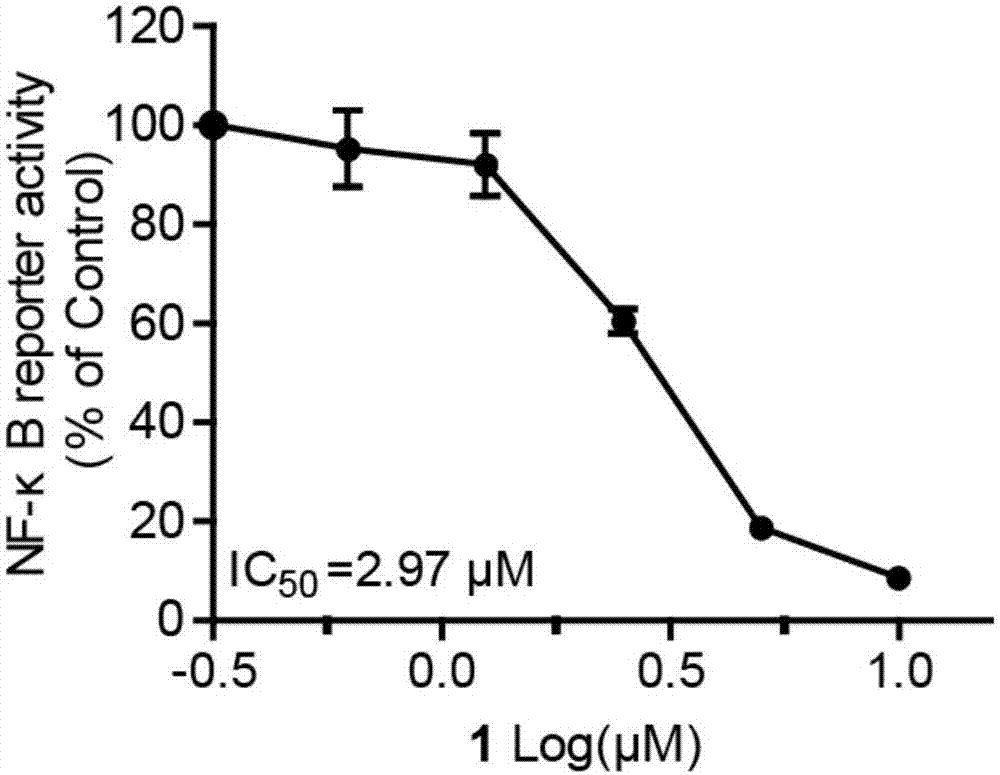
[0033] The naphthoquinone dimer Rubioncolin C (1) of the present invention utilizes SRB colorimetry to evaluate its effect on human non-small cell lung cancer cell line (A549), human liver cancer cell line (BEL-7402, HepG2 and SMMC-7721), human gastric adenocarcinoma Cell lines (BGC-823 and SGC-7901), human prostate cancer cell lines (DU145), human cervical cancer cell lines (HeLa), human breast cancer cell lines (MDA-MB-231 and MCF-7) and human glia The cytotoxic activity of 11 kinds of tumor cell lines (all cells come from Shanghai Cell Bank, Chinese Academy of Sciences) including plasmoma cell line (U251) was found to have cytotoxic activity of naphthoquinone dimer. The experimental principles, methods and results are as follows:
[0034] Experimental principle: SRB (sulfonylrhodamine B), a water-soluble protein dye, has a sulfonic acid group anion in the molecule, and can combine with basic amino acids of intracellular proteins under weakly acidic conditions. The base dis...
Embodiment 3
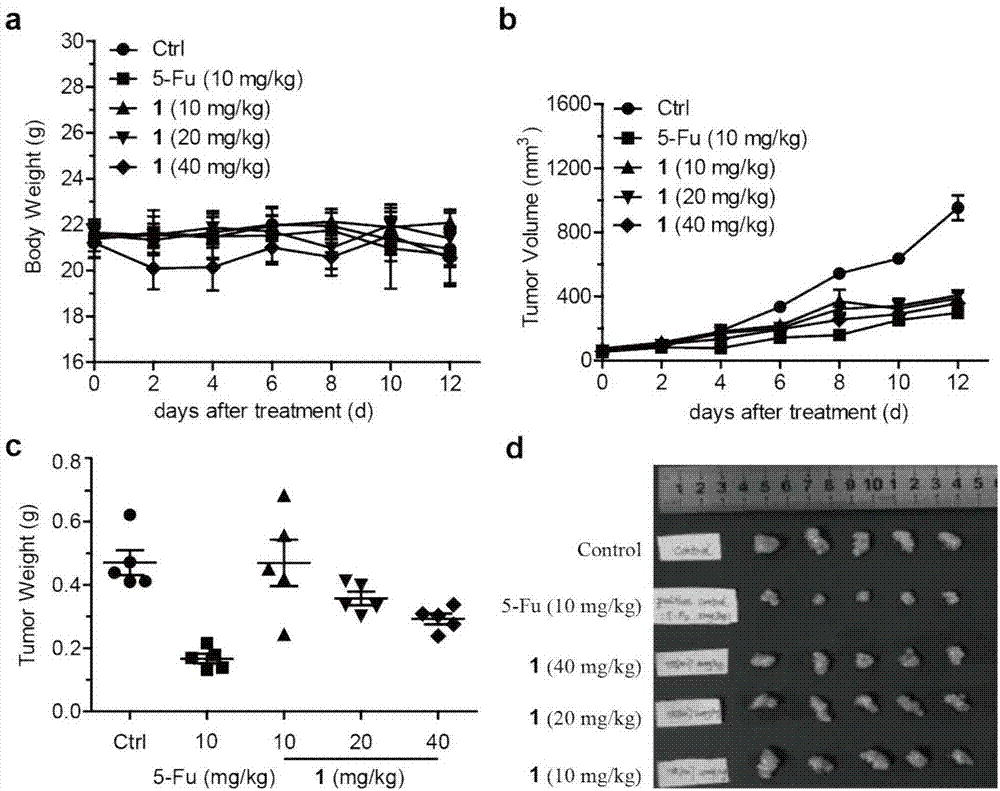
[0040] The naphthoquinone dimer Rubioncolin C(1) of the present invention is evaluated for its anti-tumor activity in vivo by using the human liver cancer HepG2 nude mouse xenograft model, and the results show that the naphthoquinone dimer has better anti-tumor activity in vivo. The experimental methods and results are as follows:
[0041] Human liver cancer cells HepG2 (from Shanghai Cell Bank, Chinese Academy of Sciences) were prepared with serum-free DMEM medium to a concentration of 1×10 5 cells / mL cell suspension, which was inoculated subcutaneously in the left armpit of BABL / c nude mice, and after 7 days of growth, a tumor-bearing mouse model was formed. The well-inoculated tumor-bearing mice were randomly divided into groups, and injected intraperitoneally with naphthoquinone dimer Rubioncolin C (1) (40 mg / kg, 20 mg / kg and 10 mg / kg), administered once every other day, and sacrificed 14 days after administration All animals were stripped and weighed, the tumor inhibitio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com