A kind of electrochemical catalyst for oxygen evolution reaction and its preparation and application
An oxygen evolution reaction and electrochemical technology, applied in the field of electrochemistry, can solve the problems of unsuitability for large-scale production, slow kinetic process, high price, etc., and achieve excellent catalytic electrochemical oxygen evolution reaction effect, improve efficiency, and catalytic performance. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation and electrochemical characterization of an electrochemical catalyst for oxygen evolution reaction with a molar ratio of cobalt to molybdenum of 8:1
[0038] 1) Co(NO 3 ) 2 ·6H 2 O 0.291 g (1 mmol) and 50 mg of polyvinylpyrrolidone (average molecular weight 10,000 Daltons) were added to 6 mL of water, and stirred rapidly at room temperature for 15 min to obtain a uniform pink solution. Then 2 mL of hydrazine hydrate was added dropwise, and stirred vigorously at room temperature for 15 min (using a stirrer at 300 rpm), to obtain a pink suspension. The obtained pink suspension was transferred to a polytetrafluoroethylene-lined autoclave, heated at 160° C. for 24 h, and then naturally cooled to room temperature to obtain a pink solid. After washing 3 times with water and 2 times with ethanol, and centrifuging at 6000rpm for 15 minutes at a high speed, the solid in the lower layer was collected, and then dried in an oven at 60°C. The red solid obtai...
Embodiment 2
[0046] Example 2. Preparation and electrochemical characterization of an electrochemical oxygen evolution catalyst with a molar ratio of cobalt to molybdenum of 4:1
[0047] Same as Example 1, just the Co(NO in step 1) 3 ) 2 ·6H 2 O is replaced by CoCl 2 ·6H 2 O. The stirring 15min in step 1) was changed to stirring 30min. 6000rpm high-speed centrifugation is replaced by stirring 8000rpm high-speed centrifugation.
[0048] Same as Example 1, just change the ammonium thiomolybdate in step 2) into 10.4mg. The heating at 200°C in step 2) was replaced with heating at 180°C. 6000rpm high-speed centrifugation is replaced by stirring 8000rpm high-speed centrifugation.
[0049] By controlling the proportion of raw materials, the black solid is an electrochemical oxygen evolution catalyst with a molar ratio of cobalt and molybdenum elements of 4:1.
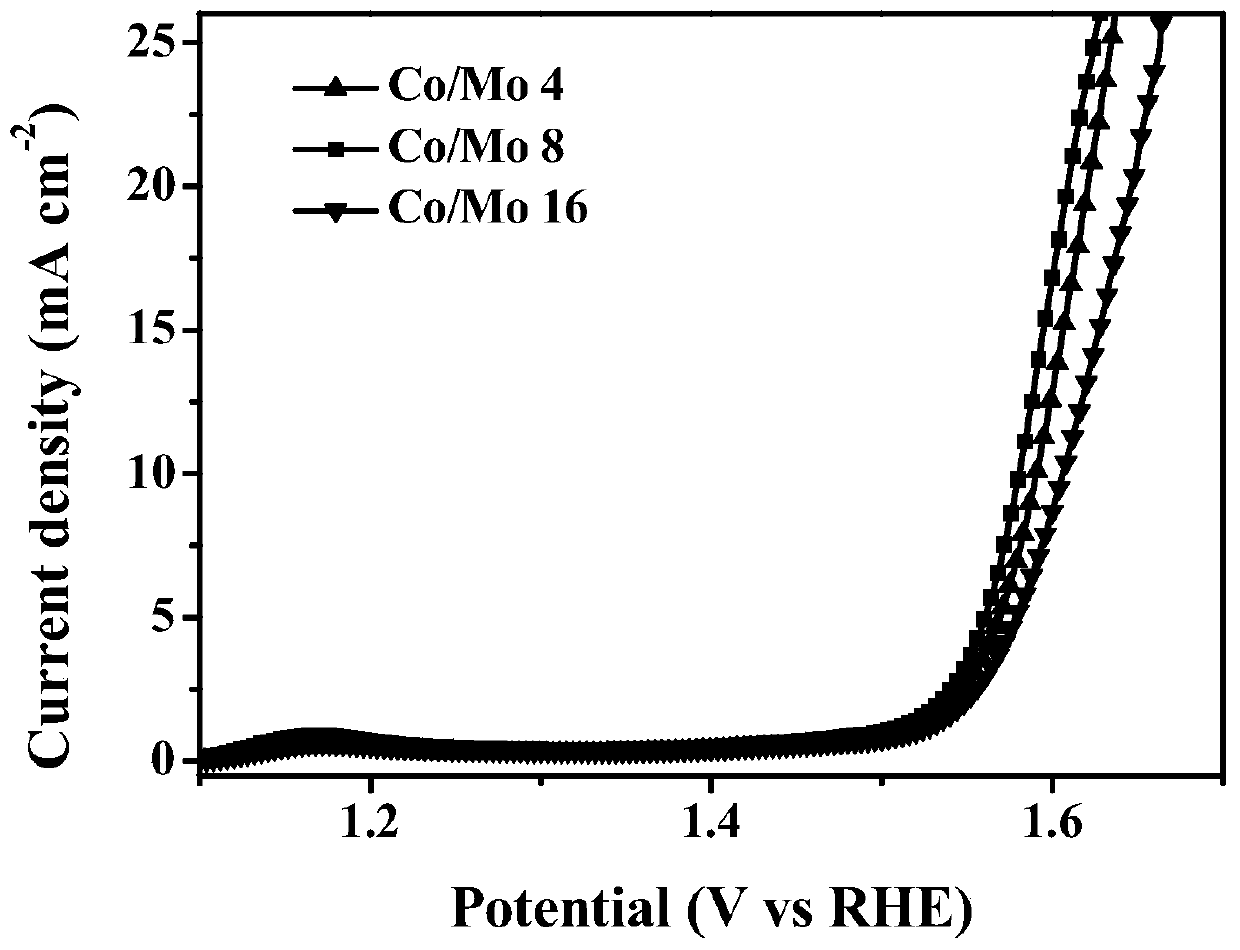
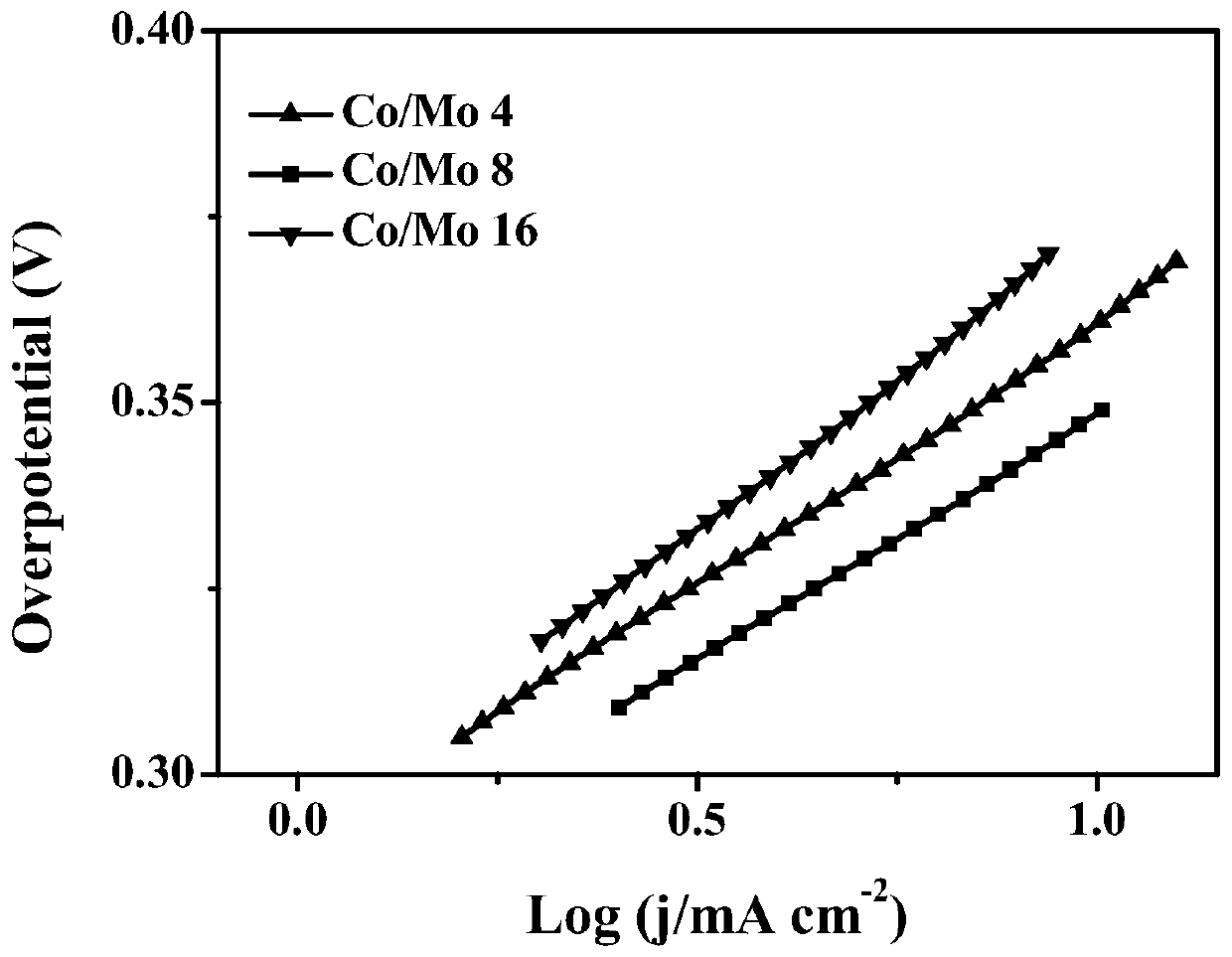
[0050] Depend on figure 1 In a and d, it can be seen that the electrochemical oxygen evolution catalyst with a molar ratio of c...
Embodiment 3
[0053] Example 3. Preparation and electrochemical characterization of an electrochemical oxygen evolution catalyst with a molar ratio of cobalt to molybdenum of 16:1
[0054] Same as Example 1, just the Co(NO in step 1) 3 ) 2 ·6H 2 O to CoSO 4 ·7H 2 O. Stirring for 15 minutes was replaced by stirring for 10 minutes. 6000rpm high-speed centrifugation is replaced by stirring 5000rpm high-speed centrifugation.
[0055] Same as Example 1, just change the ammonium thiomolybdate in step 2) into 2.6mg. The heating at 200°C in step 2) was replaced with heating at 210°C. 6000rpm high-speed centrifugation is replaced by stirring 5000rpm high-speed centrifugation.
[0056] The obtained black solid is the electrochemical oxygen evolution catalyst with a molar ratio of cobalt to molybdenum of 16:1.
[0057] Depend on figure 1 In (c) and (f), it can be seen that the electrochemical oxygen evolution catalyst with a molar ratio of cobalt to molybdenum of 16:1 has a lamellar structur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com