A kind of cobalt borophosphate material and its preparation method and application
A cobalt borophosphate material and technology of cobalt borophosphate, applied in chemical instruments and methods, physical/chemical process catalysts, electrolytic components, etc., can solve the problems of large initial potential, poor stability, high overpotential, etc., and achieve low overpotential , particle size is small, the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
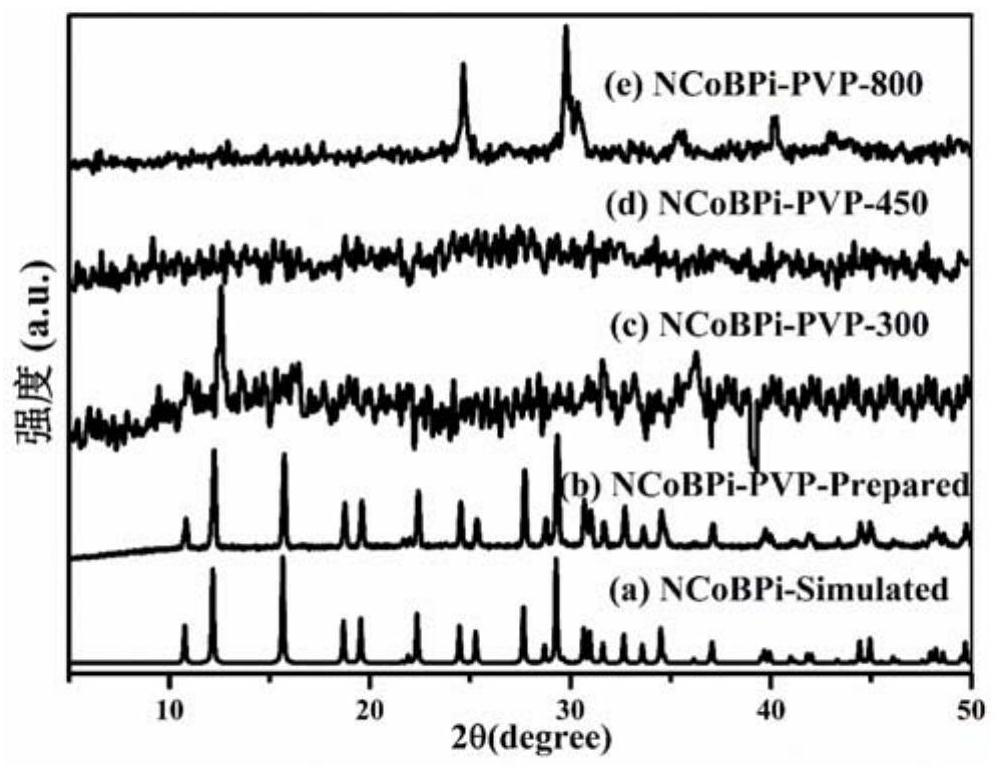
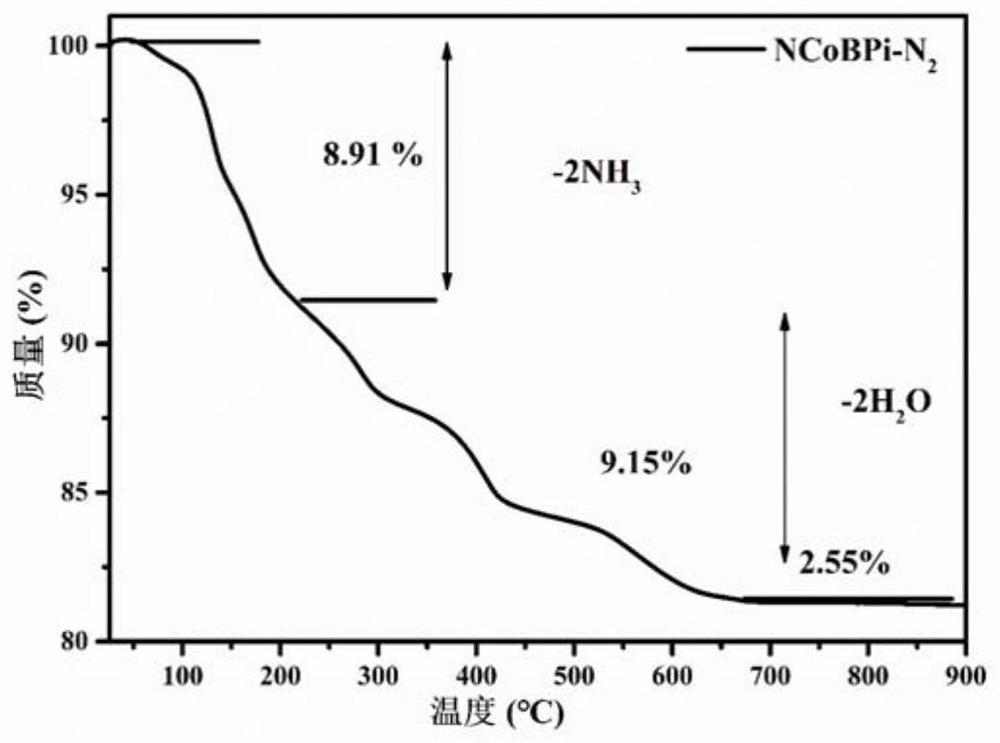
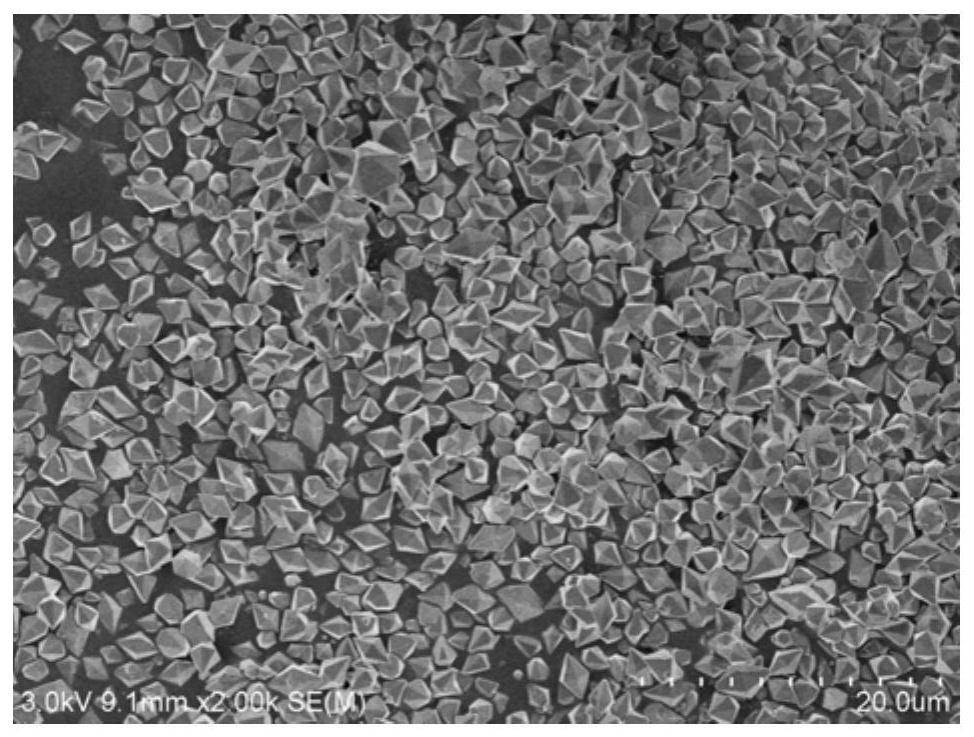
[0037] The preparation of a novel cobalt borophosphate crystal material comprises the following steps: mixing cobalt nitrate, sodium borate, ammonium phosphate, and polyvinylpyrrolidone (12KDa) with water in a molar ratio of 1.5:0.45:0.4:1.5:7, heating and stirring Evenly, the initial gel mixture is obtained, and the pH of the gel mixture is measured to be 1.0; the resulting initial gel mixture is transferred to a polytetrafluoroethylene hydrothermal reaction kettle, sealed and placed in a box-type resistance furnace, at a temperature of 160 ° C Crystallize for 8 hours, cool to room temperature, filter, and wash five times with a mixed solvent of water and ethanol to obtain H(NH 3 ) 2 Co(H 2 O) 2[BP 2 o 8 ] crystalline material, namely the sample NCoBPi-PVP.
[0038] The above crystalline material was placed in a watch glass and dried in an oven at 50°C for 1 hour, and then calcined at 450°C for 1 hour in a nitrogen atmosphere to obtain the N-doped cobalt borophosphate ca...
Embodiment 2
[0040] The preparation of a novel cobalt borophosphate crystal material comprises the following steps: mixing cobalt chloride, sodium metaborate, diammonium hydrogen phosphate, and polyvinylpyrrolidone (60KDa) with water in a molar ratio of 1.0:0.45:0.4:1.5:6 Mix, heat and stir evenly to obtain an initial gel mixture, and the pH of the gel mixture is measured to be 2.0; the resulting initial gel mixture is transferred to a polytetrafluoroethylene hydrothermal reaction kettle, sealed and put into a box-type resistance furnace, Crystallize at 180°C for 10 hours, cool to room temperature, filter, and wash four times with a mixed solvent of water and ethanol to obtain H(NH 3 ) 2 Co(H 2 O) 2 [BP 2 o 8 ] crystalline material, namely the sample NCoBPi-PVP.
[0041] The above crystal material was placed in a watch glass and dried in an oven at 80°C for 1 hour, and then calcined at 200°C for 2 hours in a nitrogen atmosphere to obtain the N-doped cobalt borophosphate catalyst mater...
Embodiment 3
[0043] The preparation of a novel cobalt borophosphate crystal material comprises the following steps: mixing cobalt acetate, boric acid, ammonium dihydrogen phosphate, and polyvinylpyrrolidone (150KDa) with water in a molar ratio of 2.0:0.5:0.6:1.8:10, heating Stir evenly to obtain an initial gel mixture, and the measured pH of the gel mixture is 4.0; transfer the obtained initial gel mixture to a polytetrafluoroethylene hydrothermal reaction kettle, seal it and put it in a box-type resistance furnace, and heat it at 220°C Crystallize at high temperature for 20 hours, cool to room temperature, filter, and wash three times with a mixed solvent of water and ethanol to obtain H(NH 3 ) 2 Co(H 2 O) 2 [BP 2 o 8 ] crystalline material, namely the sample NCoBPi-PVP.
[0044] The above crystal material was placed in a watch glass and dried in an oven at 80°C for 2 hours, and then calcined at 800°C for 4 hours in a nitrogen atmosphere to obtain the N-doped cobalt borophosphate cat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com