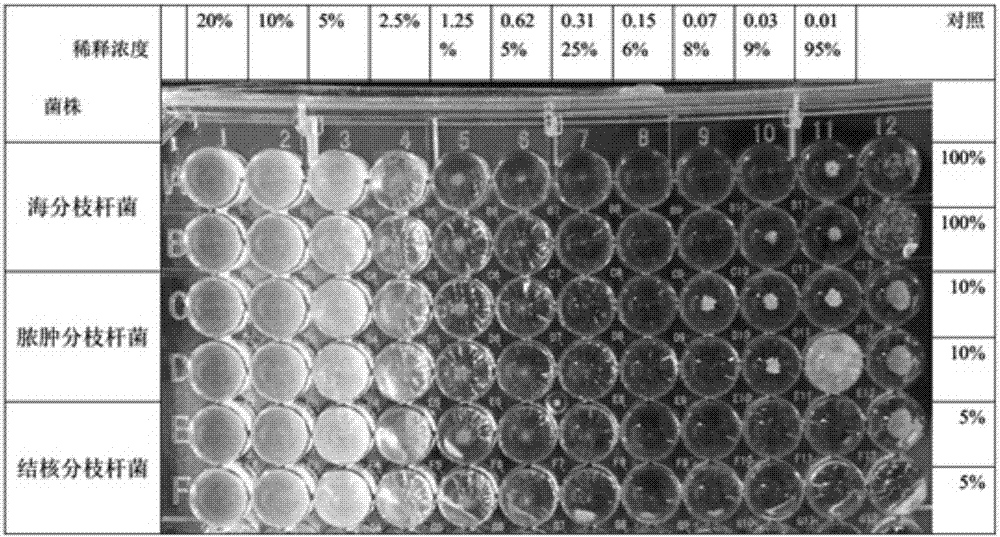
Medicine composite used for treating pulmonary tuberculosis
A composition and a technology for pulmonary tuberculosis are applied in the field of pharmaceutical compositions for the treatment of pulmonary tuberculosis, can solve the problems of poor water solubility, limited application in the field of biomedicine, etc., and achieve the effects of remarkable effect, great clinical application value and social benefit, and suitability for industrialized production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The preparation of embodiment 1 chitosan oligosaccharide (enzymatic method)
[0019] Chitosan 10g (deacetylation degree 95%, moisture 4.85%, ash content 0.5%, viscosity-average molecular weight 100,000, purchased from Jinan Haidebei Marine Engineering Co., Ltd.) was dissolved with acetic acid 333ml, and the chitosan concentration was 0.03g / ml, add 5ml of papain (enzyme content 45u) for hydrolysis, adjust the pH value to 6.1 after hydrolysis for 20mim, continue to degrade for 6h at 50°C, inactivate the enzyme for 15min at 100°C, centrifuge to get the supernatant, freeze-dry to obtain oligomeric chitosan Sugar 9.57g. Through HPLC test, it is confirmed that it is chitosan oligosaccharide, and its average molecular weight is 1200.
Embodiment 2
[0020] The preparation of embodiment 2 pharmaceutical composition
[0021] Chitosan oligomeric purity ≥ 98%, molecular weight 600-4000, embodiment 1 makes following each composition raw material all can buy:
[0022]
[0023]
[0024] Take a 200ml beaker, add 100ml of depyrogenated distilled water, then add 5g chitosan oligosaccharide, 1g Chuanbei extract, 0.8g Prunella vulgaris extract, 0.8g Bletilla striata extract, 0.5g Duheng extract, 0.5g Fenghancao extract 1. 0.5g of acanthus extract, fully stirred to make it evenly mixed, boiled for 5min, cooled to room temperature 25°C, and filtered to remove insoluble matter to obtain 109.1g of a pharmaceutical composition solution.
[0025] The pharmaceutical composition prepared in Example 2 was used in the following experiments.
Embodiment 3
[0026] Example 3 Acute Toxicity Test
[0027] Experimental animals: 70 Kunming mice, weighing 17-22 g, male and female, were purchased from Shanghai Slack Experimental Animal Co., Ltd.
[0028] Grouping of animals: The male and female were randomly divided into 7 groups with 10 animals in each group, one of which was a blank control group.
[0029] Experimental sample: prepared in Example 2.
[0030] Dosage determination: Dose design is 3ml / kg, 10ml / kg, 50ml / kg, 100ml / kg.
[0031] Administration: one-time intragastric administration: fasting for 3-5 hours before administration, fasting for 1-2 hours after administration, and fasting without water. Dosing in batches was adopted, the first batch of administration was given to the control group, 3ml / kg group, and 10ml / kg group. After administration, it was determined that the set dose was appropriate, and then 50ml / kg group and 100ml / kg group were administered. After administration, observe continuously for more than 7 days, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com