Preparation method of 4-acetoxyl-2-methyl-2-butene-1-aldehyde
A technology of acetoxy and diacetoxy, which is applied in the field of preparation of 4-acetoxy-2-methyl-2-butene-1-aldehyde, can solve the problem of unsuitable batch preparation, complicated reaction routes, Respond to high-risk problems, achieve low-cost and easy-to-obtain conversion rate, increase waste water production, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
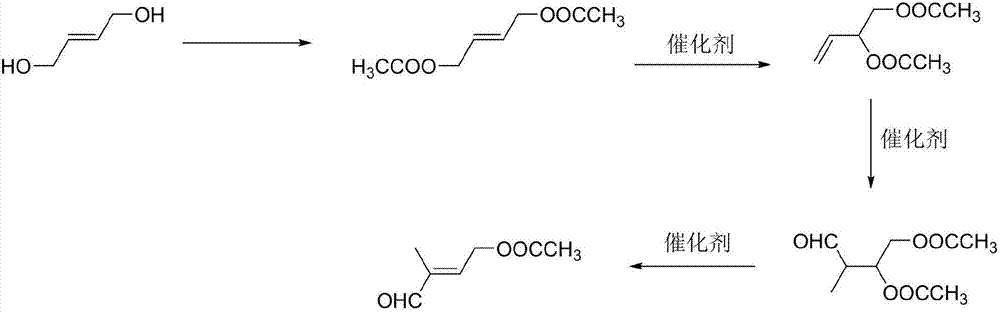
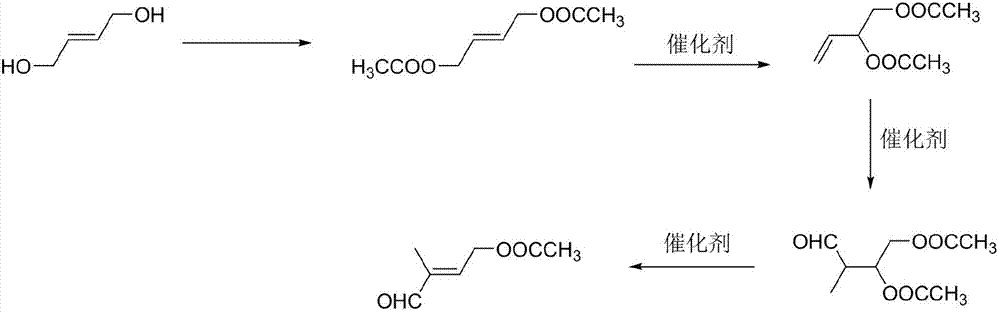
[0028] (1) Preparation of 1,4-butenediol diethyl ester: add 120 g of acetic acid to 60 g of 1,4-butenediol, heat up to reflux for 2 hours, separate water, follow the reaction in gas phase, wait for 1,4- Stop the reaction after the peak area of butenediol is ≤0.5%, recover acetic acid under reduced pressure, and obtain 1,4-butenediol diethyl ester with a yield of 81.5% through rectification.
[0029] (2) Preparation of 3,4-diacetoxy-1-butene: add 0.1g of cuprous chloride to 60g of 1,4-butenediol diethyl ester, heat up to 130°C for reaction, and follow the reaction in gas phase , stop the reaction until the content of 1,4-butenediol diethyl ester remains unchanged, remove cuprous chloride by filtration, and obtain 3,4-diacetoxy-1-butene through rectification, with a yield of 45.2 %.
[0030] (3) Preparation of 2-methyl-3,4-diacetoxy-1-butanal: add 60g 3,4-diacetoxy-1-butene and 0.03g tri(tri Phenylphosphine) carbonyl rhodium hydride, exhaust the air in the autoclave, then fe...
Embodiment 2
[0033] (1) Preparation of 1,4-butenediol diethyl ester: add 120 g of acetic anhydride to 60 g of 1,4-butenediol, heat up to reflux for 2 hours, separate water, follow the reaction in gas phase, wait for 1,4 The reaction was stopped after the peak area of -butenediol was less than or equal to 0.5%, the acetic acid was recovered under reduced pressure, and 1,4-butenediol diethyl ester was obtained by rectification with a yield of 83.5%.
[0034] (2) Preparation of 3,4-diacetoxy-1-butene: Add 0.1g of cuprous bromide to 60g of 1,4-butenediol diethyl ester, heat up to 130°C for reaction, and follow the reaction in gas phase , stop the reaction until the content of 1,4-butenediol diethyl ester remains unchanged, remove cuprous chloride by filtration, and obtain 3,4-diacetoxy-1-butene through rectification, with a yield of 37.5 %.
[0035] (3) Preparation of 2-methyl-3,4-diacetoxy-1-butanal: add 60g 3,4-diacetoxy-1-butene and 0.03g rhodium oxide in the autoclave, Exhaust the air ...
Embodiment 3
[0038] (1) Preparation of 1,4-butenediol diethyl ester: add 120 g of acetyl chloride to 60 g of 1,4-butenediol, heat up to reflux for 2 hours, separate water, follow the reaction in gas phase, wait for 1,4 The reaction was stopped after the peak area of -butenediol was less than or equal to 0.5%, the acetic acid was recovered under reduced pressure, and 1,4-butenediol diethyl ester was obtained by rectification with a yield of 83.4%.
[0039](2) Preparation of 3,4-diacetoxy-1-butene: Add 0.1g of cuprous iodide to 60g of 1,4-butenediol diethyl ester, heat up to 130°C for reaction, and follow the reaction in gas phase , stop the reaction until the content of 1,4-butenediol diethyl ester remains unchanged, remove cuprous chloride by filtration, and obtain 3,4-diacetoxy-1-butene through rectification, with a yield of 33.8 %.
[0040] (3) Preparation of 2-methyl-3,4-diacetoxy-1-butanal: add 60g 3,4-diacetoxy-1-butene and 0.03g rhodium acetylacetonate to the autoclave , exhaust ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com