Gene vaccine for preventing and treating tumors and preparation method and application of gene vaccine
A genetic vaccine and gene technology, applied in the field of preparation and design of genetic vaccines, can solve the problems of low antigen presentation efficiency, complex vaccine components, poor integration, etc., to improve long-term effect and stability, and improve antigen presentation efficiency , the effect of reducing the free time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
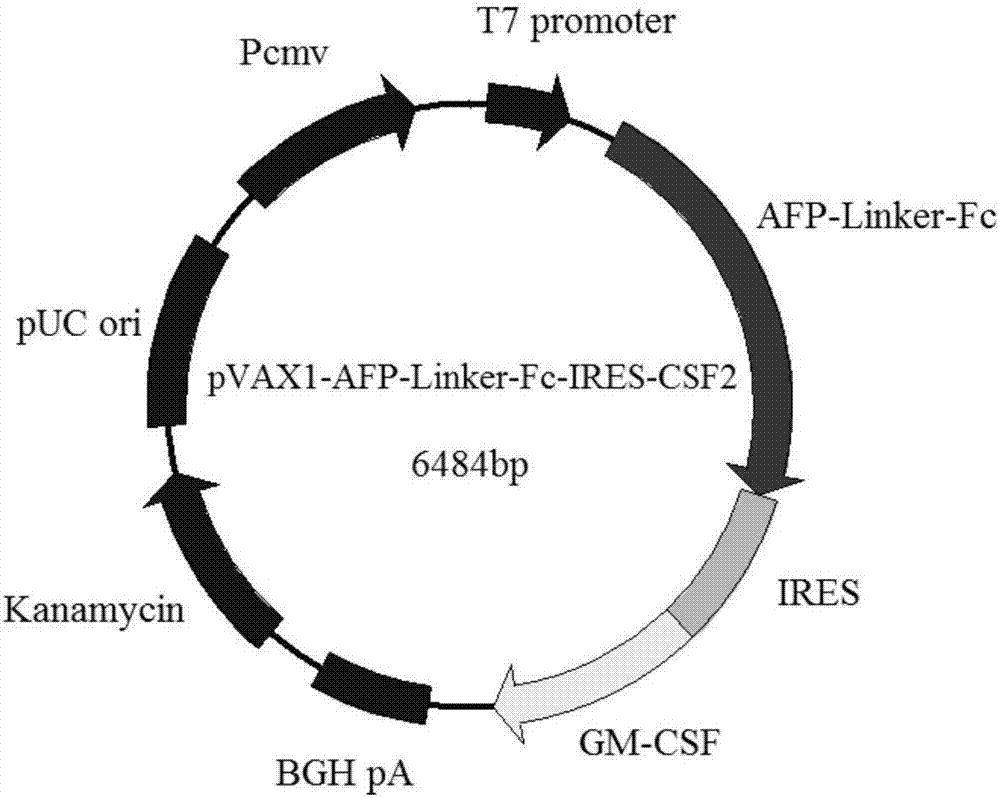
[0068] Embodiment 1: Construction of vaccine plasmid pVAX1-AFP-linker-Fc-IRES-CSF2
[0069] Plasmids and strains
[0070] The recombinant plasmid H3088pAV-MCMV-AFP containing the AFP gene sequence was synthesized and constructed by Shanghai Yingjun Biotechnology Co., Ltd., and the GenBank sequence number of AFP is: NM_001134.2.
[0071] The cDNA clones containing pVAX1 eukaryotic expression vector and CSF2 were purchased from Changsha Yingrun Biotechnology Co., Ltd., and the GenBank sequence number of CSF2 is: BC113999.1.
[0072] The plasmid vector pIRES2-EGFP containing the IRES sequence was donated by the laboratory of Zhejiang University School of Medicine.
[0073] The Fc segment includes the CH2, CH3 and hinge regions of the human IgG1 heavy chain region. The sequence was synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd., and the nucleotide sequence is NO: 2 in the sequence listing.
[0074] 1.1 PCR amplification of gene sequence AFP-linker.
[0075] The AFP gen...
Embodiment 2
[0120] Example 2: In vitro expression detection of vaccine plasmid pVAX1-AFP-Linker-Fc-IRES-CSF2
[0121] 2.1 Plasmid-transfected cells
[0122] Observe under a microscope, and transfect when HEK293 cells grow to more than 80%. Set up a group of negative control group and a group of plasmid transfection experiment group, wherein DNA of negative control group adopts pVAX1 empty plasmid, DNA of plasmid transfection experiment group adopts pVAX1-AFP-Linker-Fc-IRES-CSF2, uses PolyJet of SignaGen company TM DNA transfection reagent in vitro: The above-mentioned plasmid DNA is used to transfect HEK293 cells, and the specific steps are as follows (the following steps take the 6cm culture dish system as an example): 1. 60 minutes before transfection, use 2mL of 10% fetal bovine serum (Sijiqing) DMEM (Hyclone) medium was used to replace the cells; 2. Use DMEM serum-free high-glucose medium to dilute plasmid DNA and PolyJetTM transfection reagent, using DNA (μg): PolyJetTM (μL) ratio ...
Embodiment 3
[0130] Example 3. Vaccine inhibits the proliferation of HepG2 liver cancer cells
[0131] 3.1 Vaccine transfection inhibition test
[0132] 1.5 x 10 per well 3 A HepG2 cell was seeded in a 24-well plate and injected into 500 μL DMEM high-glucose medium (containing 10% FBS) for culture. After culturing for one day, transfection was carried out. The control group and the experimental group were set up in each group, and each group was set up with 3 replicate wells. The plasmid DNA in the control group was pVAX1 empty plasmid, and the plasmid DNA in the experimental group was constructed with pVAX1-AFP-Linker-Fc. -IRES-CSF2 via PolyJet from SignaGen TMDNA in vitro transfection reagent The above plasmid DNA was transfected into HepG2 cells. The specific steps were according to the instructions of the reagents. A complex of 5 g of plasmid DNA and 1 μL of liposomes was added to each well of a 96-well plate for transfection, and culture was continued for 96 h in a low serum state ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com