Synthesis method of ceftazidime
A technology of ceftazidime and synthetic method, which is applied in the field of synthesis of biomedicine and chemical technology, can solve problems such as low product yield, and achieve the effects of high yield, reduced ring damage, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
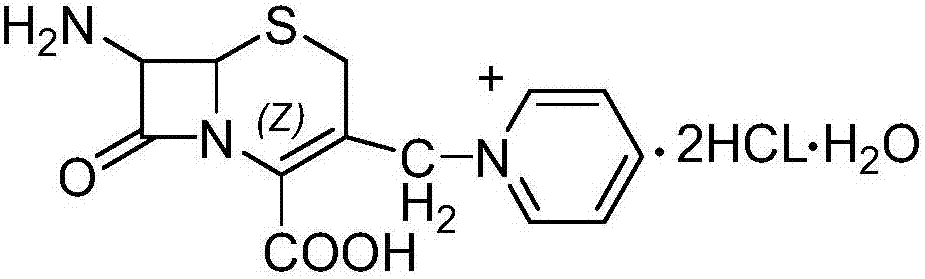
[0041] Example 1: 7--ACP·2HCl·H 2 Preparation of O
[0042]In a dry 500ml four-necked flask, add 50g (0.184mol) of 7-ACA and 38g of hexamethyldisilazane amine, heat and reflux for 8 hours, then cool down to 8°C±2°C, add 10g (0.11mol) of aniline, and stir After 0.5 hours, add 42g (0.21mol) iodotrimethylsilane, stir and react for 3 hours, and measure the residue of 7-ACA by HPLC. , N-dimethylformamide, stirred and reacted for 0.5 hours, then below -10°C, added 18g (0.228mol) of pyridine, stirred and reacted for 4 hours under temperature control, cooled to -35°C after the reaction, and added dropwise 15% HCl / isopropanol solution 150ml, after the dropwise addition, stir the reaction for 30 minutes, then add 100ml of purified water, stir well, separate layers, in CH 2 Cl 2 Add 40ml of purified water to the phase for extraction once more, combine the water phases, then add 500ml of acetone to the water phase, control the temperature at 5°C to 10°C, stir, grow crystals for 1 hou...
Embodiment 2
[0043] Example 2: 7--ACP·2HCl·H 2 Preparation of O
[0044] In a dry 500ml four-neck flask, add 50g (0.184mol) of 7-ACA and 38g of hexamethyldisilazane amine, heat and reflux for 8 hours, cool down to 8°C±2°C, add 10g (0.093mol) of 2-formazolamide Aniline, stirred for 0.5 hours, added 40g (0.225mol) iodotrimethylsilane, stirred and reacted for 3 hours, HPLC determined 7-ACA residues, after passing the test, the temperature dropped to -3°C ~ -5°C, added 7ml (0.12mol ) tetrahydrofuran and 40ml N,N-dimethylacetamide, stirred and reacted for 1.0 hour, and then added 18g (0.228mol) of pyridine at a temperature below -5°C, and reacted with temperature controlled stirring for 4 hours. Add 120ml of 15% HCl / isopropanol solution, after the dropwise addition, stir the reaction for 30 minutes, then add 120ml of purified water, stir well, separate layers, and 2 Cl 2 Then add 50ml of purified water to extract once, combine the water phase, add 600ml of acetone to the water phase, control...
Embodiment 3
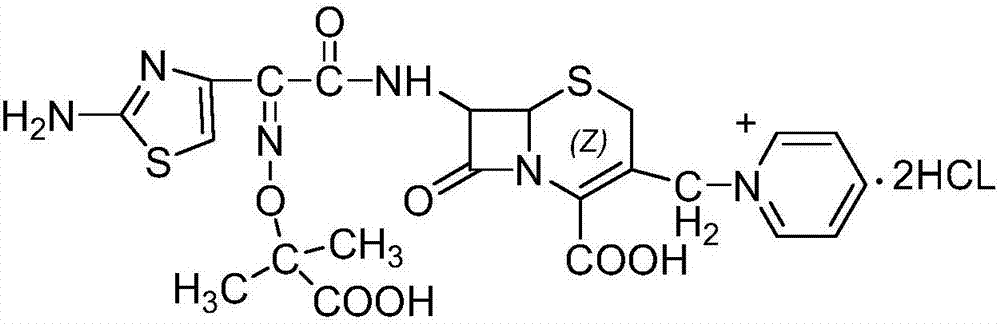
[0045] Embodiment 3: the preparation of ceftazidime dihydrochloride
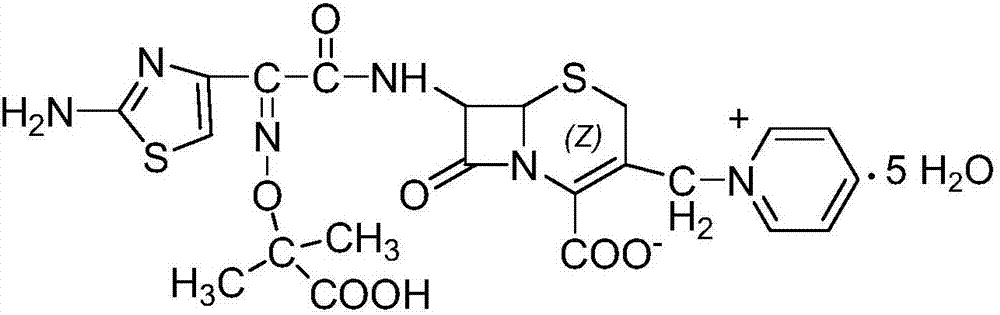
[0046] In a dry 1000ml four-necked bottle, add 7-ACP·2HCl·H 2 O 40g (0.104mol), dichloromethane 400ml, ceftazidime active ester 55g (0.115mol), cool down at 0-5°C, add 40g (0.4mol) of triethylamine dropwise, add dropwise for about 15min, and time the reaction for 6h after the dropwise addition , the reaction was completed, filtered, washed with 100ml of dichloromethane, and dried at 45°C to obtain 62g of ceftazidime axetil as an intermediate.
[0047] In another 1000ml four-necked bottle, add 62g of ceftazidime axetil, 50g of formic acid, 50ml of concentrated hydrochloric acid, control the temperature at 20°C-25°C, stir for 3-4h for hydrolysis reaction, after the reaction is completed, add 600ml of acetone dropwise, crystallize, and grow the crystal for 1h. Cool down to 5°C-10°C, grow crystals for 1.5h, filter, wash with acetone, and vacuum-dry at 45°C to obtain 52g of ceftazidime dihydrochloride with a yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com