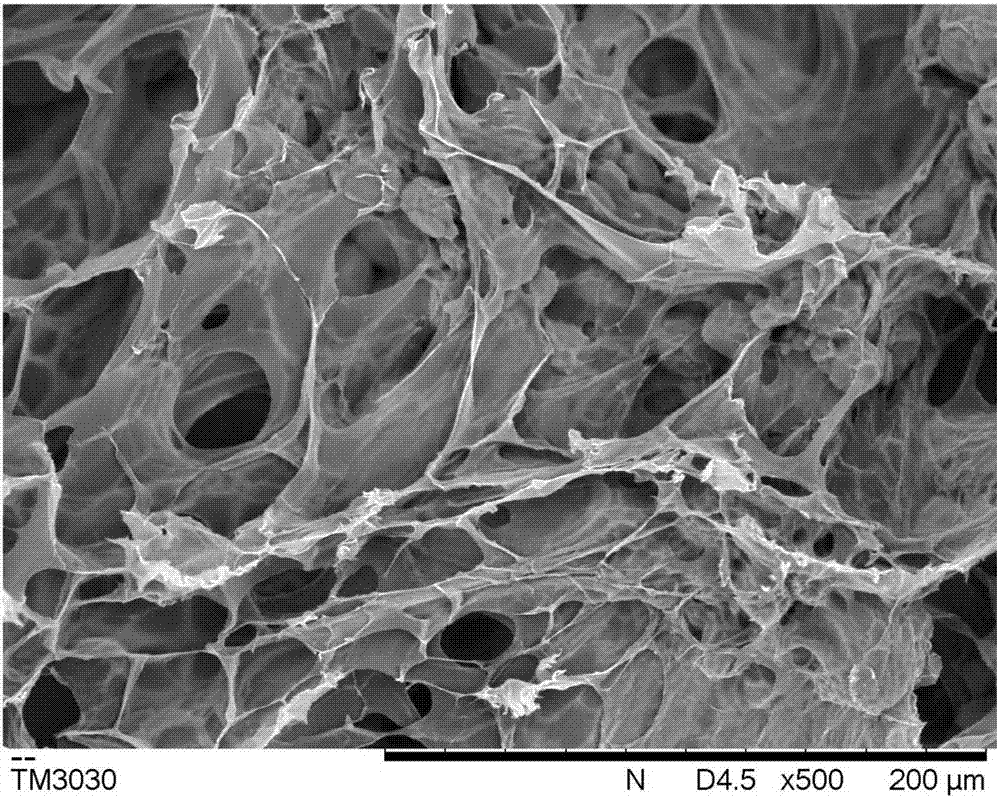
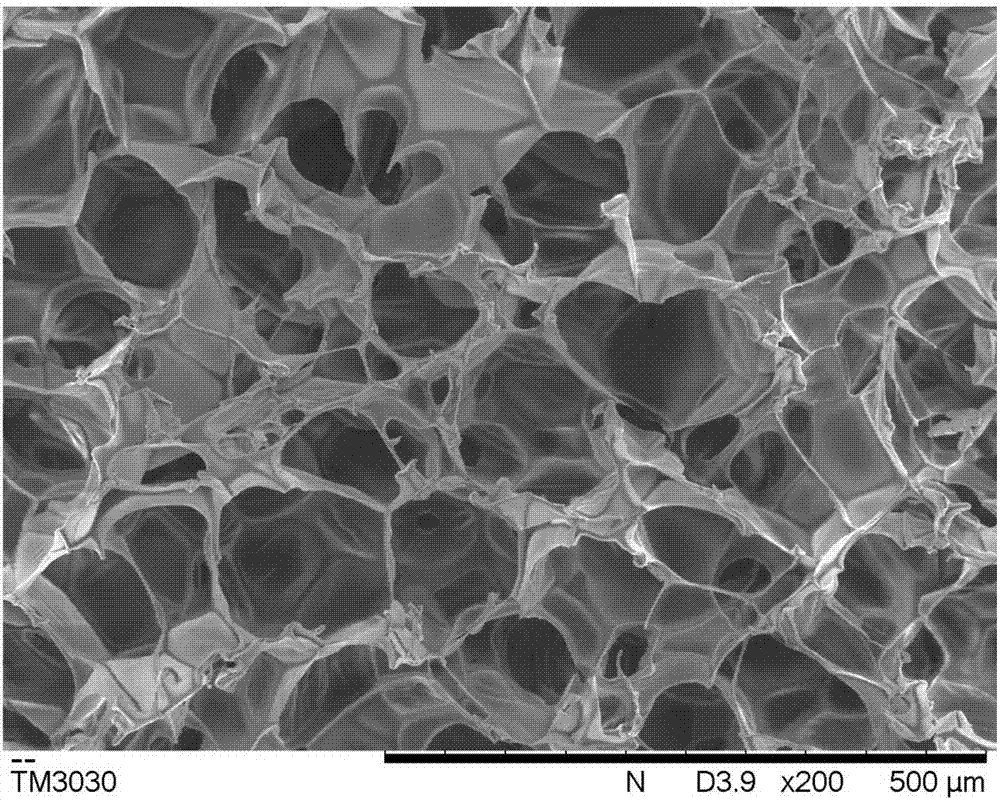
Sanguinarine/gelatin microspheres-loaded composite hydrogel support and preparation method and application thereof
A technology of compounding hydrogel and sanguinarine, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and drug combinations, can solve the problems of easy failure of sanguinarine oxidation, and avoid the The effect of drug resistance, wide antibacterial range, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of preparation method of the composite hydrogel support of loading sanguinarine / gelatin microspheres of the present embodiment, comprises the following steps:
[0039] (1) Dissolve 100 mg of sodium hyaluronate in 20 ml of deionized water, obtain a 2 mg / ml sodium hyaluronate solution on a shaker at 37 ° C and 100 r / min, and then add 10 times the amount of adipic dihydrazide ( 1g) was dissolved under magnetic stirring at a rate of 400 r / min at room temperature, and the pH after the reaction was adjusted to 6.8 with 0.1 mol / L HCl and 0.1 mol / L NaOH solution. Dissolve 192mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 132mg of 1-hydroxybenzotriazole in 1ml (dimethylsulfoxide:water=1:1) in the mixture. Add the freshly prepared mixed solution dropwise to the reaction solution adjusted to pH=6.8 and continue mixing at room temperature for 6-12 hours, maintaining the pH at 6.8. Use 0.1 mol / L HCl and 0.1 mol / L NaOH solution to adjust the pH after r...
Embodiment 2
[0045] A kind of preparation method of the composite hydrogel support of loading sanguinarine / gelatin microspheres of the present embodiment, comprises the following steps:
[0046] (1) Dissolve 100 mg of sodium hyaluronate in 20 ml of deionized water, obtain a 2 mg / ml sodium hyaluronate solution on a shaker at 37 ° C and 100 r / min, and then add 10 times the amount of adipic dihydrazide ( 1g) was dissolved by magnetic stirring at a rate of 400r / min at room temperature. The pH after the reaction was adjusted to 6.8 with 0.1 mol / L HCl and 0.1 mol / L NaOH solution. Dissolve 192mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 132mg of 1-hydroxybenzotriazole in 1ml (dimethylsulfoxide:water=1:1) in the mixture. Add the freshly prepared mixed solution dropwise to the reaction solution adjusted to pH=6.8 and continue mixing at room temperature for 6-12 hours, maintaining the pH at 6.8. Use 0.1 mol / L HCl and 0.1 mol / L NaOH solution to adjust the pH after reaction...
Embodiment 3
[0052] A kind of preparation method of the composite hydrogel support of loading sanguinarine / gelatin microspheres of the present embodiment, comprises the following steps:
[0053] (1) Dissolve 100 mg of sodium hyaluronate in 20 ml of deionized water, obtain a 2 mg / ml sodium hyaluronate solution on a shaker at 37 ° C and 100 r / min, and then add 10 times the amount of adipic dihydrazide ( 1g) was dissolved by magnetic stirring at a rate of 400r / min at room temperature. The pH after the reaction was adjusted to 6.8 with 0.1 mol / L HCl and 0.1 mol / L NaOH solution. Dissolve 192mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 132mg of 1-hydroxybenzotriazole in 1ml (dimethylsulfoxide:water=1:1) in the mixture. Add the freshly prepared mixed solution dropwise to the reaction solution adjusted to pH=6.8 and continue mixing at room temperature for 6-12 hours, maintaining the pH at 6.8. Use 0.1 mol / L HCl and 0.1 mol / L NaOH solution to adjust the pH after reaction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com