Method for controllably synthesizing alkali metal titanate nanowires and converting alkali metal titanate nanowires into titanium dioxide nanowires
A technology for synthesizing titanate and titanium dioxide, which is applied in the direction of alkali metal titanate, titanium dioxide, chemical instruments and methods, etc., can solve the problems of reducing the lifetime of photogenerated electrons, achieve reduced pressure resistance requirements, good crystallinity, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Anatase TiO 2 The molar ratio of powder to potassium hydroxide is 1:5. First, take 2 mmol of anatase in a mortar and grind for ten minutes. Then add KOH, continue to grind for about 30 minutes until it becomes viscous, transfer the mixture to a stainless steel reaction kettle with a polytetrafluoroethylene liner, and keep it at 150°C for 24h. Cool to room temperature, wash with distilled water and ethanol three times alternately. The washed product was placed in an oven at 60°C and dried for 12 hours to obtain KTiO 2 (OH) nanomaterials.
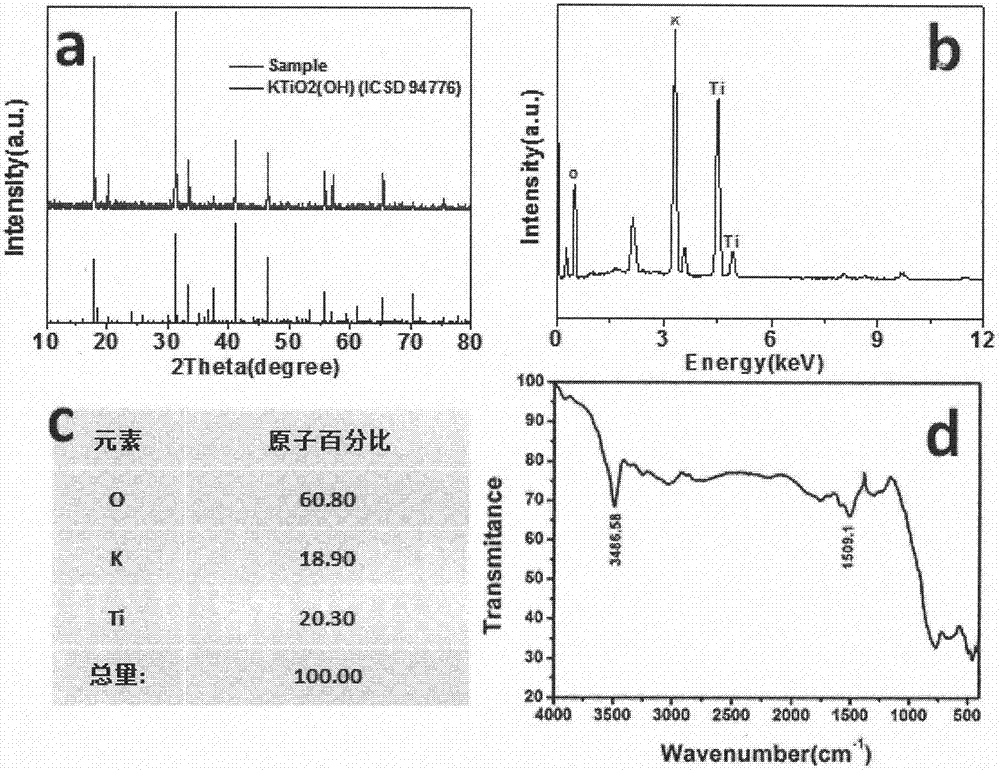
[0036] The prepared precursor KTiO 2 The X-ray powder diffraction spectrum of (OH) is as attached figure 1As shown in a, corresponding to KTiO 2 (OH) (ICSD 94776). The EDS spectrum is attached figure 1 As shown in b and 1c, the atomic ratio of K, Ti, and O is 1:1.07:3.21, which is close to the stoichiometric composition of the product. Infrared Spectrum (attached figure 1 d) at 3490cm -1 There is an O-H stretching vibration p...
Embodiment 2
[0038] With the KTiO that embodiment 1 obtains 2 (OH), calcined at 530°C for 2h in a tube furnace to obtain K 2 Ti 2 o 5 Nanowires. The collected product was dissolved in 0.1mol·L -1 Stir in hydrochloric acid solution, acidify for 2 hours, and calcinate for 2 hours at 400°C in a tube furnace to obtain the final product TiO 2 Nanowires.
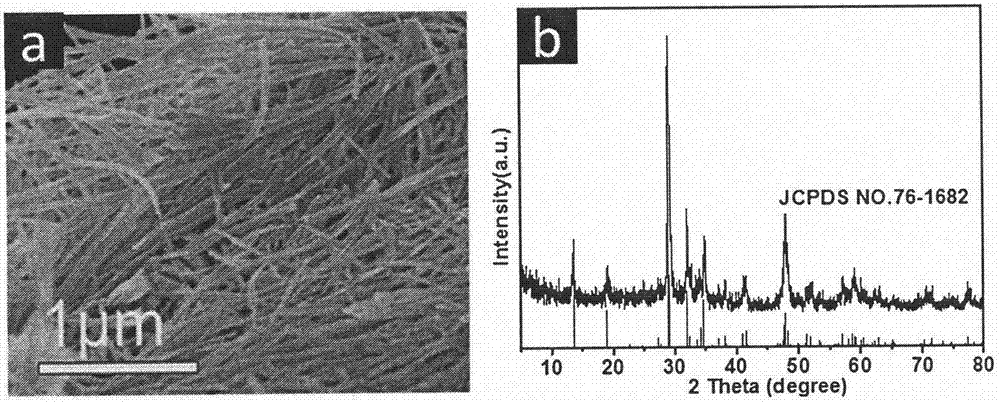
[0039] as attached image 3 As shown, K 2 Ti 2 o 5 Nanowires hold KTiO well 2 (OH) nanowire morphology, the corresponding product phase is K 2 Ti 2 o 5 (JCPDS Card 76-1628).
[0040] as attached Figure 4 As shown, K 2 Ti 2 o 5 After acidification, K is still well maintained 2 Ti 2 o 5 The nanowire morphology, and the corresponding product is H 2 Ti 2 o 5 .H 2 O (JCPDS Card 47-0124).
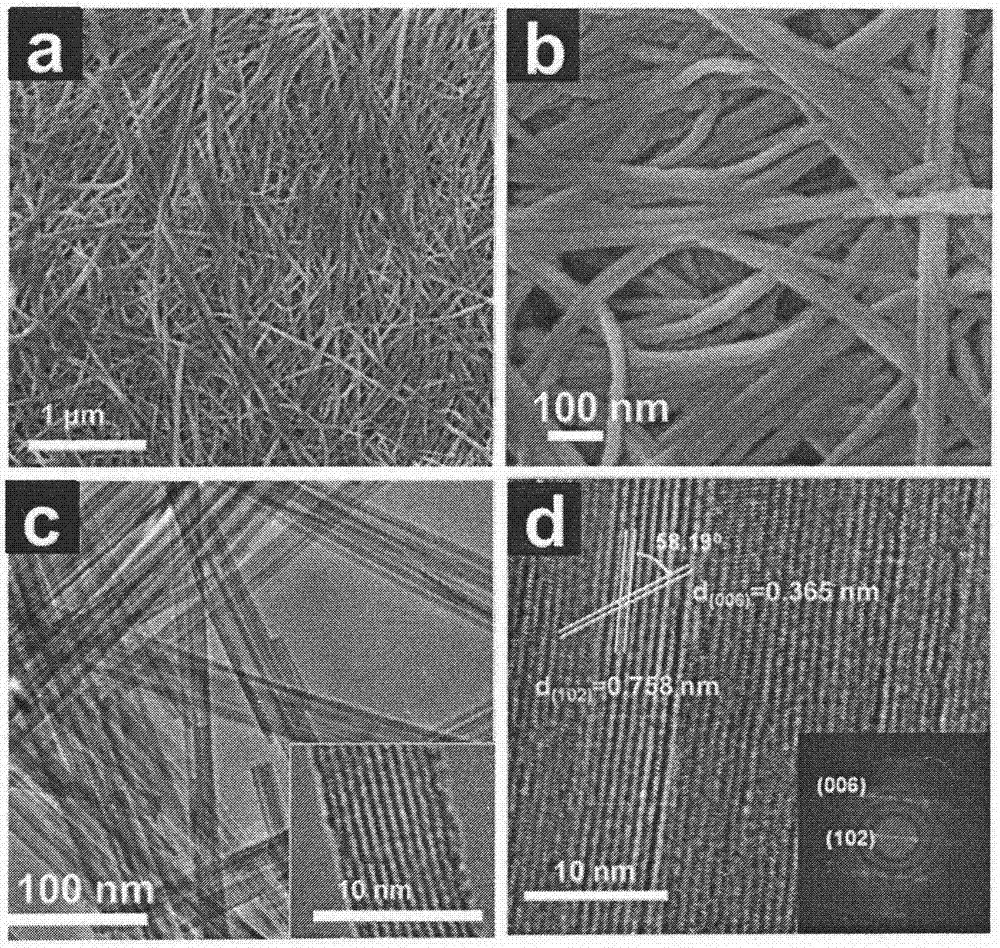
[0041] h 2 Ti 2 o 5 .H 2 O nanowires are calcined to obtain TiO 2 Nanowires. The XRD of the calcined product is as attached Figure 5 As shown, TiO in the anatase phase 2 (JCPDS Card 21-1272), and good crystallinity. attached by...
Embodiment 3
[0043] The reaction conditions are the same as in Example 1, except that the molar ratios of anatase and potassium hydroxide are set to 1:3, 1:4, and 1:6. After the reaction, it was cooled to room temperature, washed with distilled water and ethanol three times alternately, and dried in an oven at 60° C. for 12 hours to obtain a titanate precursor. The morphology and XRD of the prepared precursor are shown in the attached Figure 8 shown. When the reaction stoichiometric ratio n(KOH):n(TiO 2 ) is 1:3, only some short nanorods are formed, and it can be seen in XRD that these nanorods correspond to K 2 Ti 8 o 17 (JCPDS Card41-1100); When the reaction stoichiometric ratio is 1:4, nanowires with a diameter of about 30nm and a length of about tens of nanowires can be seen, but it can be seen as K in the XRD diffraction peak 2 Ti 8 o 17 (JCPDS Card 41-1100) and KTiO 2 (OH) (ICSD94776) mixed phase; when the reaction stoichiometric ratio is 1:6, it corresponds to the previousl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com