Crystals of N-acetylneuraminic acid ammonium salt anhydrate, and method for producing same
A technology of neuraminic acid ammonium salt and anhydrous, which is applied in the preparation of sugar derivatives, organic chemical methods, chemical instruments and methods, etc., can solve the problems of stability and storage stability, and achieve storage stability sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0083] Obtaining of Non-crystalline Amorphous Form of NeuAc Ammonium Salt
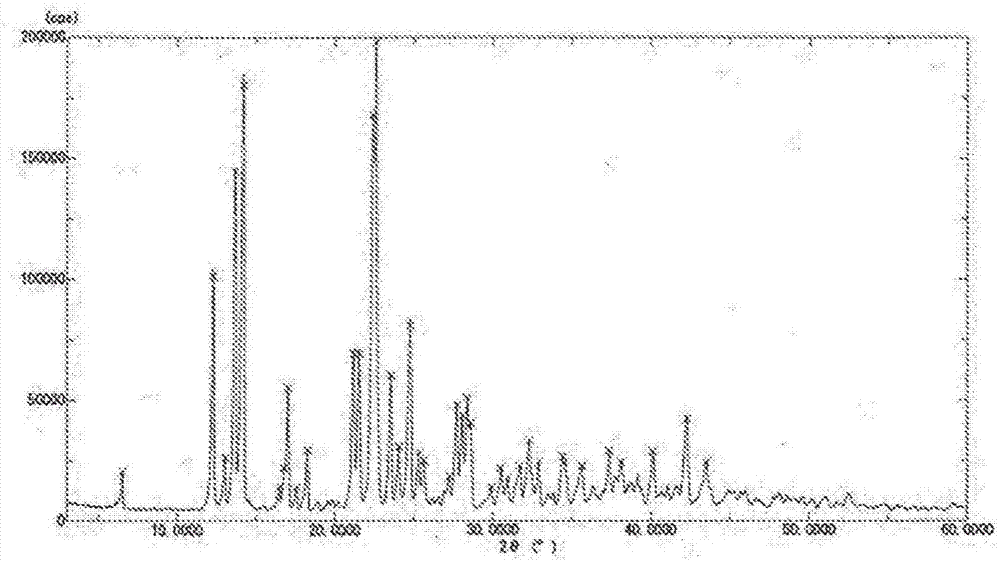
[0084] 519.4 g of NeuAc anhydrate crystals were dissolved in water, and the pH was adjusted to 6.80 using ammonia water to prepare 650 mL of an aqueous NeuAc-containing ammonium salt solution. A part of this aqueous solution was freeze-dried to obtain a white powder. When the powder was subjected to powder X-ray diffraction measurement, no X-ray diffraction peak was confirmed, so it was found that the powder was an amorphous amorphous substance.
[0085] Examples are shown below, but the present invention is not limited to the following examples.
Embodiment 1
[0087] Crystal Obtaining of NeuAc Ammonium Salt Anhydrate-1
[0088] 1546.7 g of NeuAc in terms of anhydrous matter was dissolved in water, and the pH was adjusted to 7.67 using ammonia water to obtain 3800 mL. This aqueous solution was concentrated to 1800 mL, and 500 mL of the obtained concentrated solution was used in the next step.
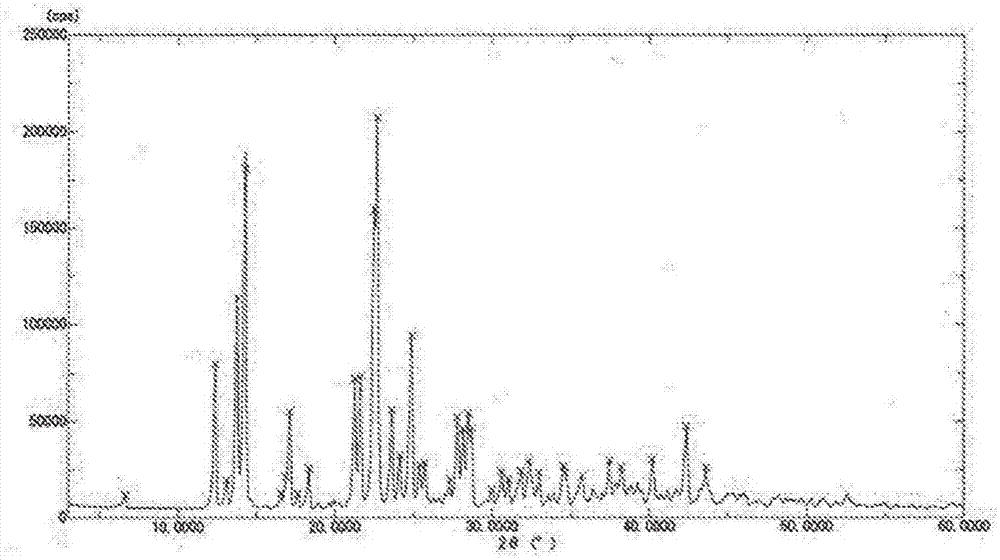
[0089] While maintaining the 500 mL concentrated solution at 45° C., 1500 mL (3 times the amount) of methanol was added dropwise thereto over 3 hours, and then 400 mL of acetone was added thereto. After aging for 1 hour, 1100 mL of acetone (final addition amount: 3 times the amount) was additionally added over 3 hours to precipitate crystals. The crystal slurry was cooled to 5° C., aged for 3 hours, and the crystals were collected by filtration, washed with 80% methanol aqueous solution, and dried under reduced pressure at 25° C. to obtain 430.8 g of crystals.
[0090] Table 1 shows the results of powder X-ray diffraction of this crystal. I...
Embodiment 2
[0102] Crystal Obtaining of NeuAc Ammonium Salt Anhydrate-2
[0103] 1475.8 g of NeuAc in terms of anhydrous matter was dissolved in water, and the pH was adjusted to 6.81 using ammonia water to prepare 4000 mL. This aqueous solution was concentrated to 1810 mL, and 40 mL of the obtained concentrated solution was used in the next step.
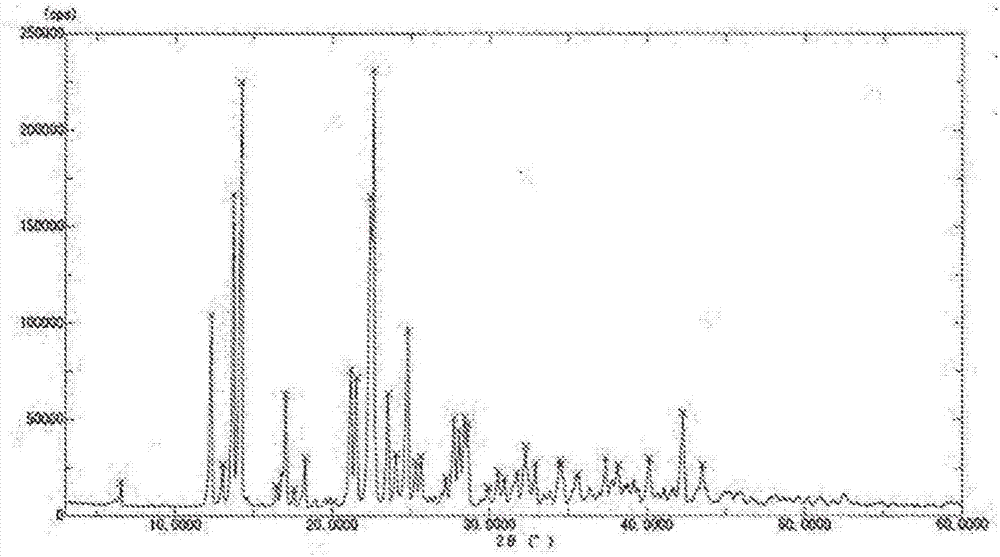
[0104] 10 mL of water was added to 40 mL of this concentrated solution to make 50 mL. While maintaining this 50 mL solution at 40° C., 40 mL of ethanol was added dropwise thereto over 1 hour. The crystal obtained in Example 1 was added as a seed crystal, and the crystal was precipitated. After aging the crystals over 9 hours, 110 mL (final addition amount: 3 times the amount) of ethanol was added dropwise over 8 hours. The crystal slurry was cooled to 10° C., aged for 3 hours, and the crystals were collected by filtration, washed with 80% ethanol aqueous solution, and dried under reduced pressure at 25° C. to obtain 32.9 g of crystals.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com