Thymopentin sustained release microsphere and its preparation method and application
A technology of slow-release microspheres and thymus, which is used in pharmaceutical formulations, peptide/protein components, medical preparations containing active ingredients, etc., and can solve the problems of large porosity and low drug loading of thymopentin sustained-release microspheres. , to achieve the effect of small total amount and high drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
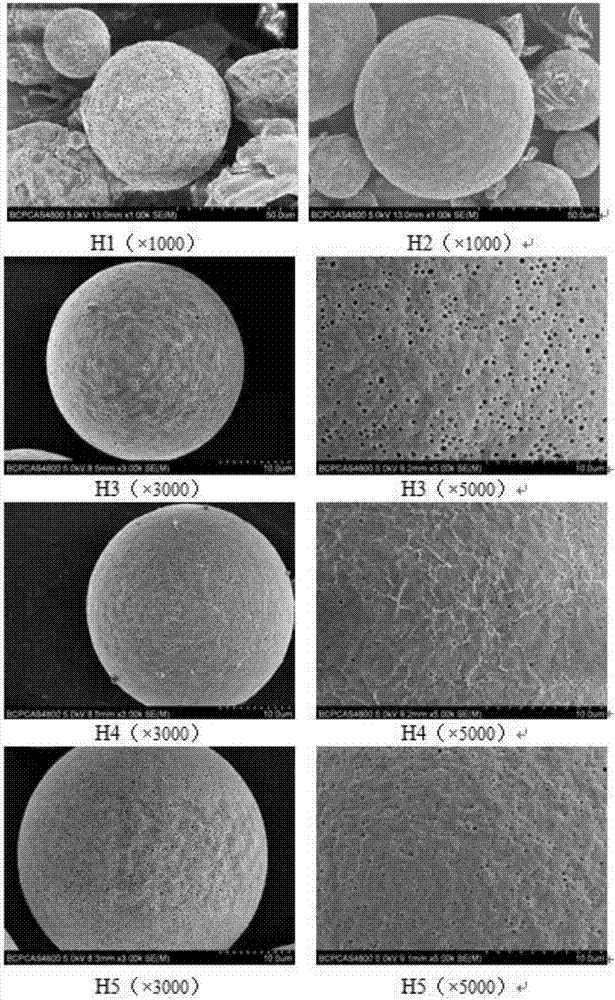
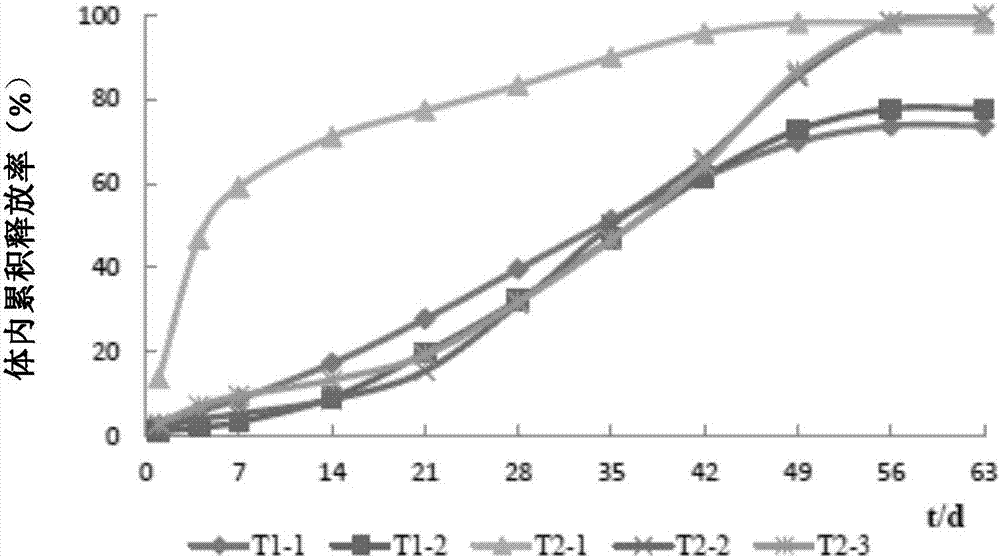
Image
Examples
Embodiment 1
[0039] Using the W / O / W double emulsion solvent volatilization method, feed the external water phase osmotic pressure regulator sodium chloride at 10, 20, 40, 60, 80 mg / mL, and polymerize the monomer of biodegradable pharmaceutical polymer material PLGA The molar ratio is 50:50 and the viscosity is 0.59dL / g. Microsphere samples were prepared as follows:
[0040]Weigh 2.0g PLGA (polymerization molar ratio 50:50, viscosity 0.59dL / g) and dissolve in 8.0mL dichloromethane to make the oil phase in double emulsion; dissolve 600mg TP-5 in 1.2mL water for injection to make inner water phase; the inner water phase is added to the organic phase of dichloromethane as the middle oil phase, placed in a high-shear emulsification disperser, and treated at 17,000rpm for 2.0min to form W / O type colostrum; with 0.5% poly Vinyl alcohol (PVA) as the emulsifier, a predetermined amount of sodium chloride (NaCl) as the osmotic pressure regulator 80mL aqueous solution as the external water phase, pou...
Embodiment 2
[0043] Using the W / O / W double emulsion solvent evaporation method, fluorescein isothiocyanate-labeled thymopentin (FITC-TP5, prepared by polypeptide solid-phase synthesis, purity ≥ 95%, kept away from light) and thymopentin ( TP-5) Feed and mix according to the weight ratio of 1:5, the concentration of sodium chloride in the external aqueous phase is 40mg / mL, the monomer polymerization molar ratio of the biodegradable pharmaceutical polymer material PLGA is 50:50, and the viscosity is 0.59dL / g. Prepare microsphere samples under dark conditions as follows:
[0044] Weigh 2.0g PLGA (polymerization molar ratio 50:50, viscosity 0.59dL / g) and dissolve it in 8.0mL dichloromethane to make the oil phase in double emulsion; dissolve 100mg FITC-TP5 and 500mg TP-5 in 1.2mL Prepare the inner water phase in water for injection; add the inner water phase to the organic phase of dichloromethane as the middle oil phase, place it in a high-shear emulsification disperser, and process it at 17...
Embodiment 3
[0047] Using the W / O / W double emulsion solvent volatilization method, the external water phase osmotic pressure regulator sodium chloride is fed at 70, 100 mg / mL, and the monomer polymerization molar ratio of the biodegradable pharmaceutical polymer material PLGA is 50:50 , the viscosity is 0.38dL / g. Prepare microsphere samples under dark conditions as follows:
[0048] Weigh 2.0g PLGA (polymerization molar ratio 50:50, viscosity 0.38dL / g) and dissolve in a mixed solvent of 6.5mL dichloromethane and 1.5mL ethyl acetate to make the oil phase in double emulsion; 600mg isothiocyanate Fluorescein-labeled thymopentin (FITC-TP5, prepared by polypeptide solid-phase synthesis, purity ≥ 95%, stored away from light) was dissolved in 1.2mL water for injection to make the inner water phase; the inner water phase was added to the oil phase In the organic phase of the mixed solvent, place it in a high-shear emulsifying disperser, and treat it at 17,000rpm for 2.0min to form W / O colostrum; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com