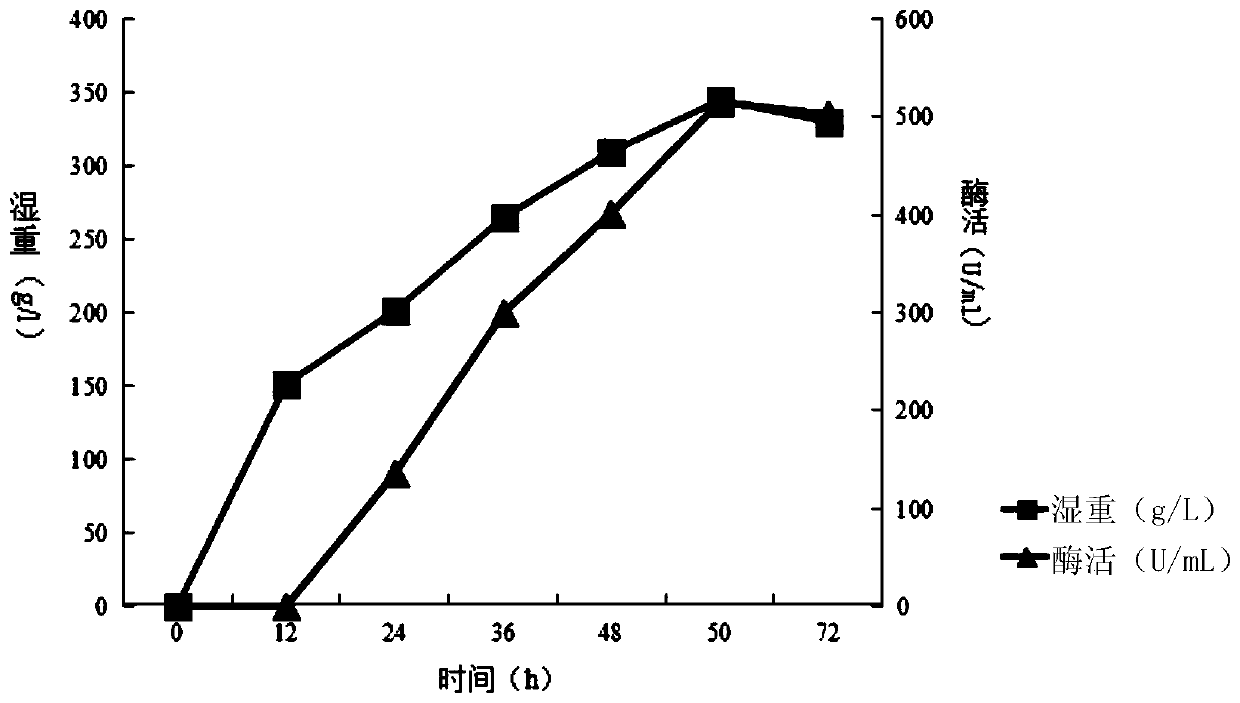
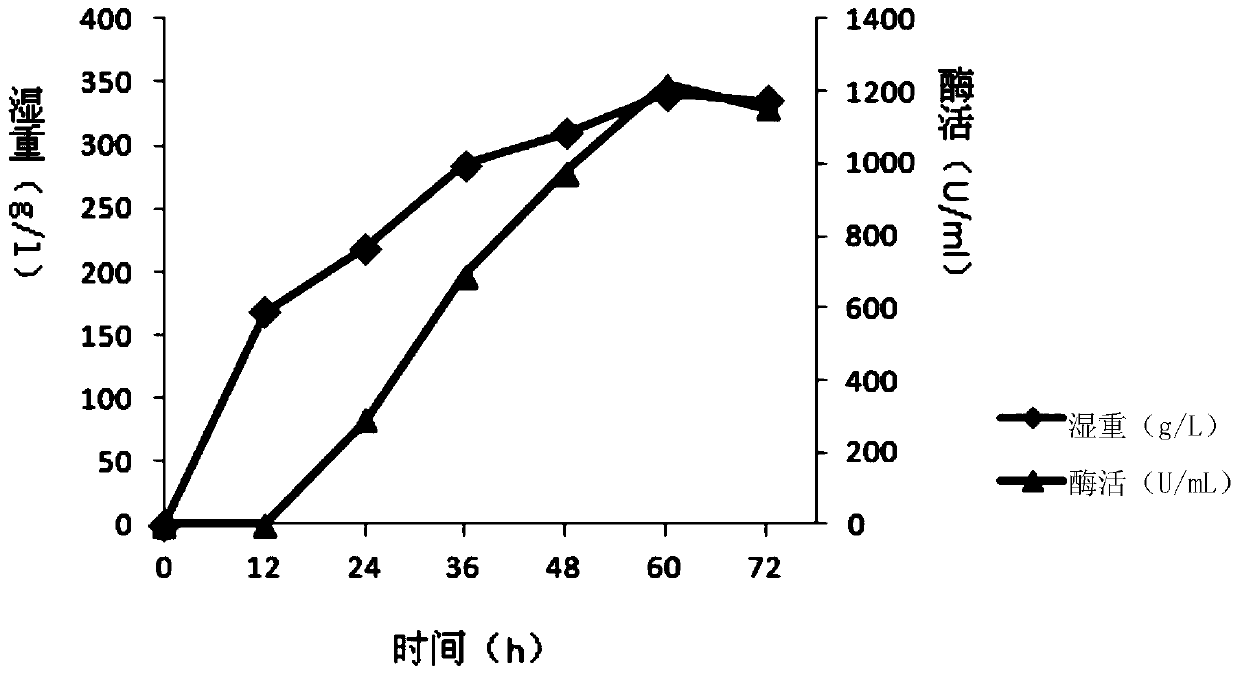
A kind of sucrose isomerase mutant and its application
A sequence and gene technology, applied in the directions of isomerase, application, enzyme, etc., can solve the problems of low sucrose isomerase ability, affecting the yield of isomaltulose, and being unfavorable for large-scale production, and achieves great application prospects and The effect of industrial value, increased expression, and enhanced specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1, discovery of mutein
[0074] On the basis of the sucrose isomerase shown in sequence 1 in the sequence listing, a mutant protein library (including single mutation, multiple mutation, etc., consisting of hundreds of thousands of proteins with specific sequences) is constructed. The enzymatic activity of each protein in the mutant protein library as a sucrose isomerase is detected, and mutant proteins with significantly increased enzyme activity are discovered, and one of the mutant proteins is shown in sequence 3 of the sequence listing.
[0075] The protein shown in sequence 1 of the sequence listing is named PRSi protein, and its coding gene is shown in sequence 2 of the sequence listing. The protein shown in sequence 3 of the sequence listing is named as PRSi-QRY protein, and its coding gene is shown in sequence 4 of the sequence listing. Compared with PRSi protein, PRSi-QRY protein has three point mutations, which are K132Q, K171R and D329Y.
Embodiment 2
[0076] Embodiment 2, the acquisition of recombinant yeast
[0077] 1. Obtaining recombinant yeast PRSi
[0078] 1. Insert the double-stranded DNA molecule shown in the sequence 2 of the sequence listing from the 1st to the 1800th nucleotide at the 5' end forwardly between the XhoI and NotI restriction sites of the pPICZA vector to obtain the recombinant plasmid pPicZa-PRSi.
[0079] 2. Digest the recombinant plasmid pPicZa-PRSi with the restriction endonuclease SacI to obtain a linearized plasmid.
[0080] 3. Introducing the linearized plasmid obtained in step 2 into Pichia pastoris X33 to obtain recombinant yeast PRSi.
[0081] 2. Obtaining recombinant yeast PRSi-QRY
[0082] 1. Insert the double-stranded DNA molecule shown in the sequence 4 of the sequence listing from the 1st to the 1800th nucleotide at the 5' end in the forward direction between the XhoI and NotI restriction sites of the pPICZA vector to obtain the recombinant plasmid pPicZa-PRSi- QRY. In the recombina...
Embodiment 3
[0085] Example 3, the preparation of protein and the enzymatic properties of protein as sucrose isomerase
[0086] 1. Protein preparation
[0087] BMGY liquid medium: the solvent is PBS buffer solution with pH 6.0 and 0.1M; the solute and its concentration are as follows: yeast extract 10g / L, tryptone 20g / L, glycerol 10g / L, YNB 13.4g / L, biotin 4×10 -4 g / L.
[0088] BMMY liquid culture medium: solvent is the PBS damping fluid of pH6.0, 0.1M; Solute and its concentration are as follows: yeast extract 10g / L, tryptone 20g / L, methanol 0.5% (volume percentage), YNB 13.4g / L, biotin 4×10 -4 g / L.
[0089] Solution I: the solvent is Tris-HCl buffer solution with pH 8.0 and 20 mM; the solute and its concentration are as follows: 20 mM imidazole, 300 mM NaCl.
[0090] Solution II: The solvent is Tris-HCl buffer solution with pH 8.0 and 20mM; the solute and its concentration are as follows: 250mM imidazole, 300mM NaCl.
[0091] The recombinant yeast is: recombinant yeast PRSi or rec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific vitality | aaaaa | aaaaa |
| Specific vitality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com