Glucose oxidase mutant and encoding gene and application thereof
A glucose oxidase and coding gene technology, applied in glucose oxidase mutants and its coding genes and application fields, can solve the problems of low expression, high production cost, hindering industrial production and application, etc., and achieve broad application prospects, Excellent thermal stability, high enzyme activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1, Site-directed Mutation of Glucose Oxidase Encoding Gene
[0070] The homology modeling of glucose oxidase GODA was carried out, and the mutation site was designed as the 82nd glutamic acid was mutated to cysteine. The mutation site was introduced by Over-lap PCR method, and it was sequenced and verified to obtain the mutant gene GODB. Primers used in Over-lap PCR are shown in Table 1:
[0071] Table 1. Mutant GODB-specific primers
[0072]
[0073] Sequencing results show that the above-mentioned Over-lap PCR amplified nucleic acid fragments are: the 5' end contains the restriction site EcoRI, the 3' end contains the restriction site Not I, and the middle part has the sequence ID of SEQ ID №: 1 in the sequence table. Nucleic acid fragment showing nucleotide sequence. Among them, the nucleic acid fragment with the nucleotide sequence shown in SEQ ID No.: 1 in the sequence listing has a total of 1746 bp, and the coding region is 1743 bp long. Shown by ac...
Embodiment 2
[0074] The preparation of embodiment 2 glucose oxidase mutant GODB
[0075] (1) Preparation of recombinant plasmid pPIC9-GODB
[0076] Carry out double enzyme digestion (Eco RI+Not I) of expression vector pPIC9; Carry out double enzyme digestion (Eco RI+Not I) of the nucleic acid fragment prepared in the above-mentioned embodiment 1 at the same time; The above-mentioned two nucleic acid fragments that cut are connected , to obtain a recombinant plasmid containing the mutant gene GODB.
[0077] The recombinant plasmid obtained above was sent for sequencing to verify the correctness of the sequence. The recombinant plasmid in which the sequence of the foreign gene inserted in the obtained plasmid is the nucleotide sequence shown in SEQ ID No. 1 is named pPIC9-GODB.
[0078] (2) Preparation of recombinant bacteria GS115 / GODB
[0079] The recombinant plasmid pPIC9-GODB was transformed into Pichia pastoris GS115 cells to obtain recombinant yeast strain GS115 / GODB. The plasmid o...
Embodiment 3
[0082] Embodiment 3 The property analysis comparison of glucose oxidase mutant GODB and wild type
[0083] (1) Enzyme activity analysis and comparison
[0084] GOD enzyme activity was determined by ultraviolet spectrophotometer. The specific method is as follows: Carry out the enzymatic reaction under the given conditions. The enzymatic reaction system is: 200 μL reaction system, including 50 μL appropriate diluted enzyme solution, 20 μL 10 mM ABTS solution, 20 μL 50 U / mL HRP solution, 90 μL hydrogen phosphate Disodium-citrate Buffer, and 20μL 1M glucose solution. Measure the growth curve of optical density with time within 3min at a wavelength of 420nm, record it every 30s, and obtain the enzyme activity according to the slope of the straight line. One enzyme activity unit (U) is defined as the amount of enzyme required to generate 1 μmol of oxidized ABTS per unit time under given conditions.
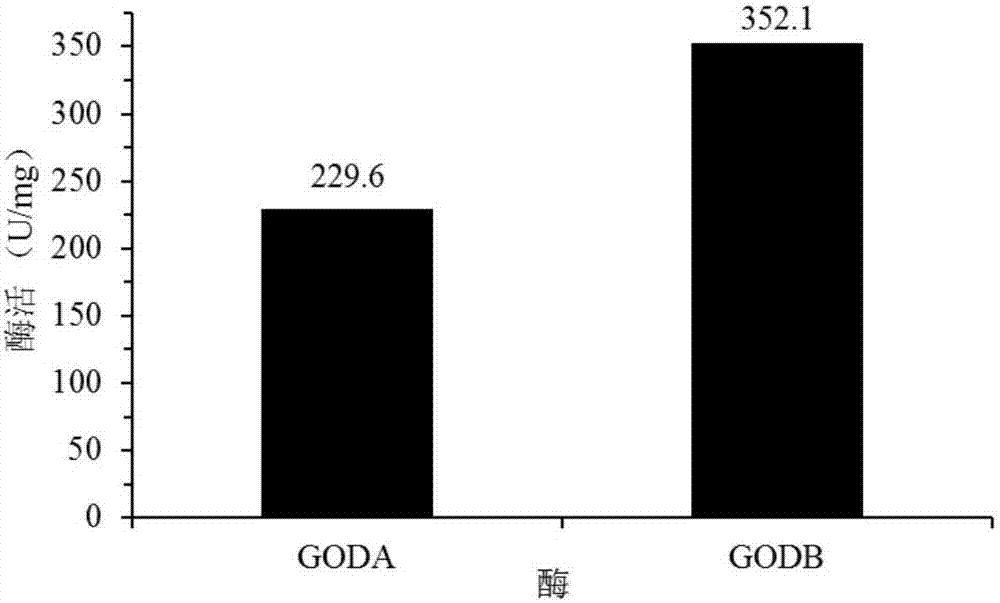
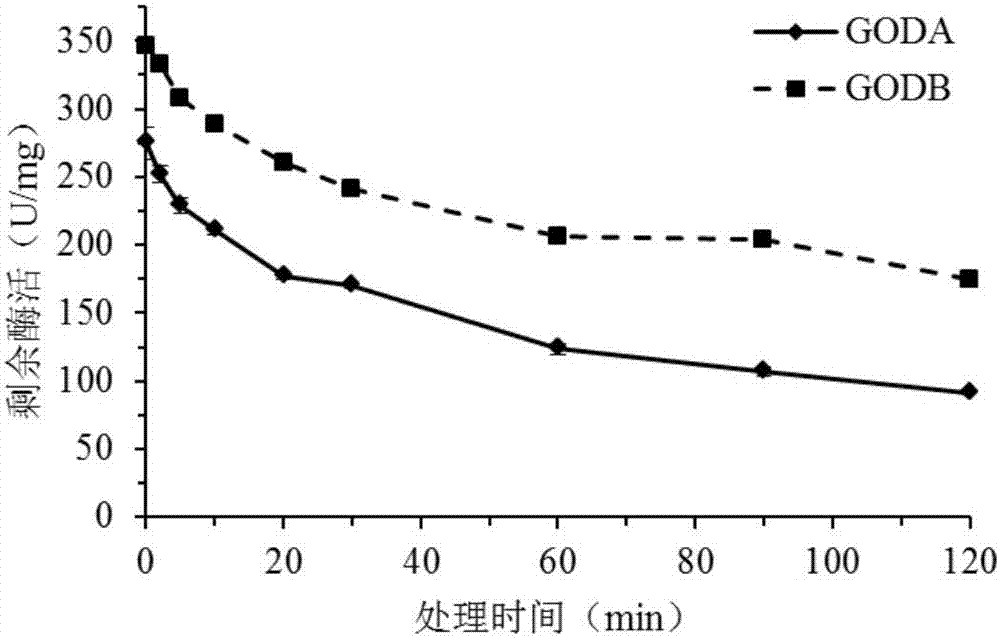
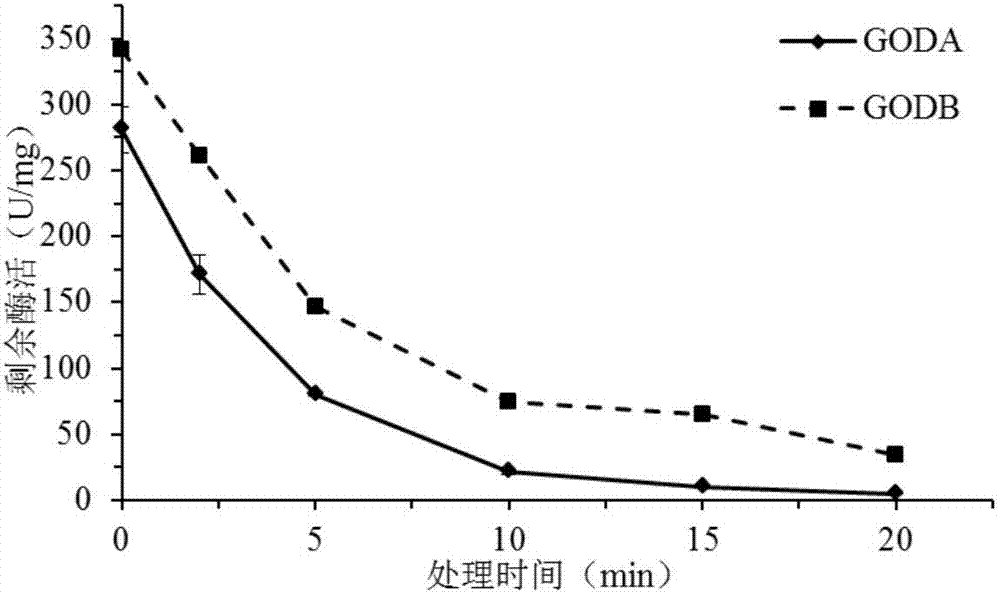
[0085] After the glucose oxidase mutant GODB prepared in the above-mentioned Ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com