Coumarin-carbazole type oxime ester compound as well as preparation method and application thereof
An ester compound, coumarin technology, applied in the field of photocurable coatings or inks, coumarin-based oxime ester-type compounds, can solve problems such as the inability to meet the research needs of visible light initiators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
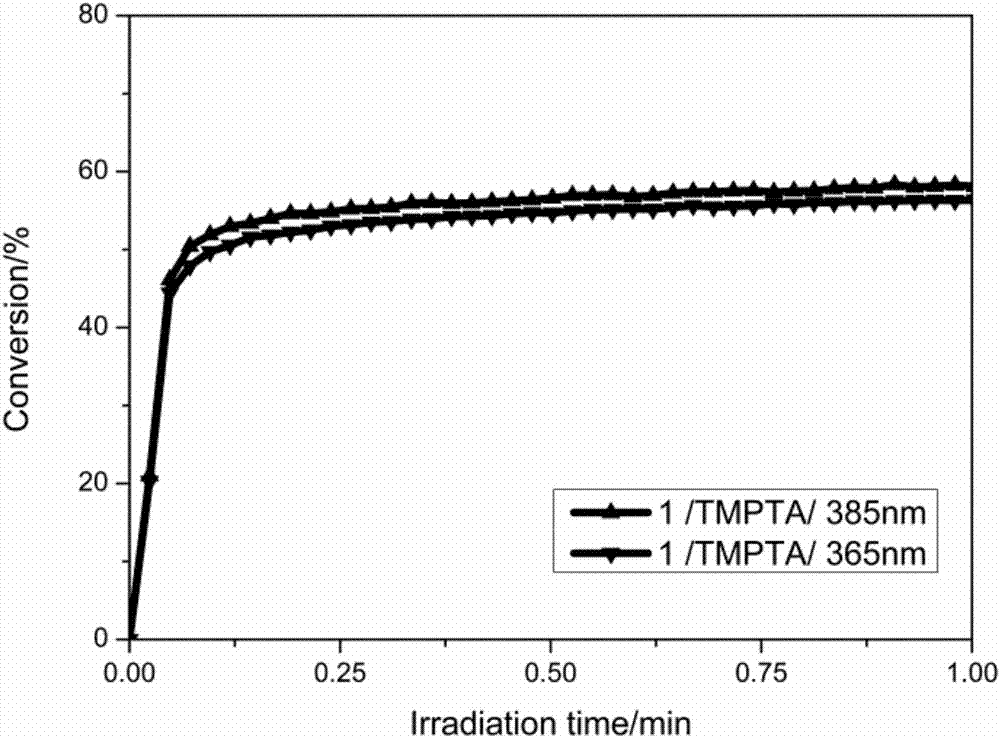
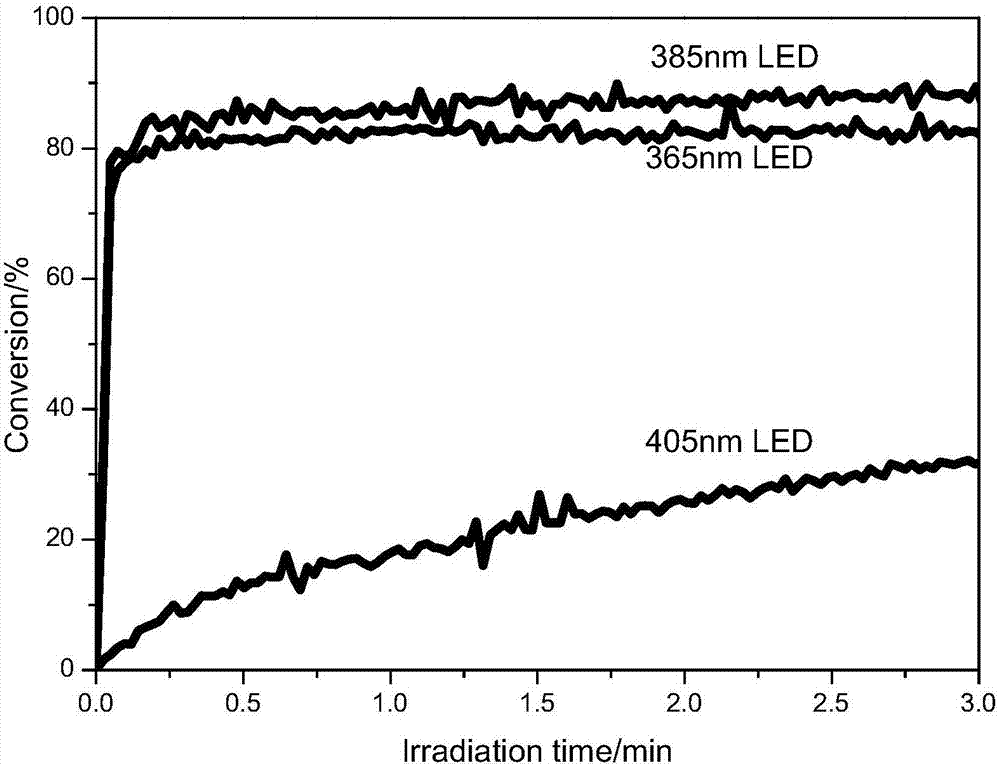
Examples
Embodiment 1
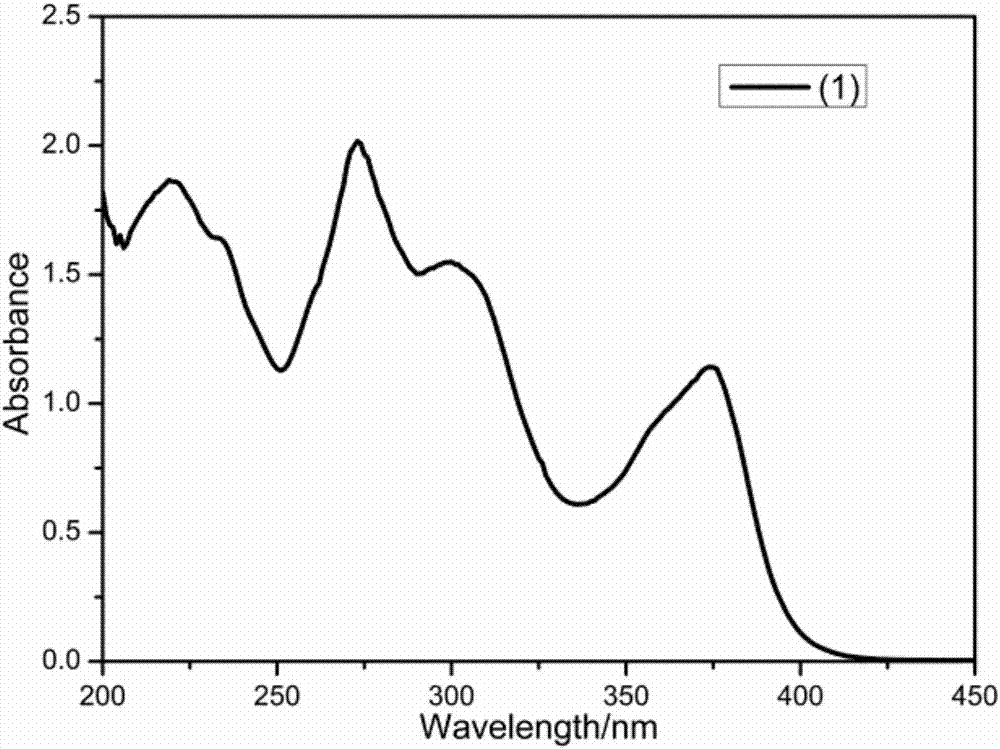
[0044] 1. Preparation and characterization of compound (1)
[0045]
[0046] The initial substance is 4-hydroxycarbazole, and the product is obtained through acylation, esterification, etc. and reaction. The used synthetic route of this preparation method is expressed as follows:
[0047]
[0048] The specific process is as follows:
[0049] Step 1, the synthesis of compound (A). in N 2 Under protection, 6.96g (0.038mol) 4-hydroxycarbazole, 9.6mL (0.076mol) ethyl acetoacetate, 1.19g (0.0038mol) BiCl were added to a 100mL round bottom flask 3 After reacting in an oil bath at 100°C for 72 h under vigorous stirring, 500 mL of absolute ethanol was added to the reaction mixture, ultrasonically dispersed, filtered, and the filter cake was washed several times with ethanol to obtain an off-white solid, which was vacuum-dried at room temperature to obtain the product ( 1) 5.84g Yield: 61.7%. The product can be directly used in the next reaction.
[0050] Step 2, the synthe...
Embodiment 2
[0067] Embodiment two: preparation and characterization of compound (2)
[0068]
[0069] Compound (2) was prepared by the same preparation method as in the above-mentioned Example 1, the only difference being that p-isovaleryl chloride was used instead of n-butyryl chloride as the raw material. The yield of the target molecule in this example was 66.3%.
[0070] The structure of the product was confirmed by nuclear magnetic resonance spectroscopy, and the specific characterization results are as follows:
[0071] 1 H NMR (400MHz, CDCl 3 )δ8.83(s, 1H), 7.95(t, J=8.0Hz, 1H), 7.52(dd, J=8.6, 2.7Hz, 1H), 7.28(d, J=8.7Hz, 1H), 7.16( s,1H),6.07(s,1H),4.05(t,J=7.4Hz,2H),2.38(s,3H),1.92–1.79(m,3H),1.34–1.11(m,15H),0.79 (dt,J=13.5,7.0Hz,7H). 13 C NMR (101MHz, CDCl 3 )δ188.82,159.34,152.29,149.02,143.13,142.86,142.73,127.37,126.63,125.57,124.25,121.82,119.98,111.50,110.62,109.23,108.29,105.25,46.84,38.46,35.88,29.83,27.66,23.24,22.51 , 21.91, 18.26, 16.11, 12.94, 9.79.
Embodiment 3
[0072] Embodiment three: preparation and characterization of compound (3)
[0073]
[0074] Compound (3) was prepared by the same preparation method as in the above-mentioned Example 1, the only difference being that benzoic anhydride was used instead of acetic anhydride as the raw material. The productive rate of present embodiment target molecule is 80.2%,
[0075] The structure of the product was confirmed by mass spectrometry, and the specific characterization results are as follows:
[0076] HRMS (M+H) for C32H25F3NO3S4: 656.0624 (calculated), 656.0619 (experimental); (M+Na) for C32H24NaF3NO3S4: 678.0443 (calculated), 678.0449 (experimental).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com