Catalyst for catalyzing saccharides to synthesize 5-hydroxymethyl furfural and method of catalyzing saccharides to synthesize the same
A technology of hydroxymethylfurfural and catalyst, which is applied in the field of catalyzing the synthesis of 5-hydroxymethylfurfural from sugar, and can solve the problem of low catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] The present invention also provides a kind of preparation method of the catalyst of above-mentioned catalytic sugar synthesis 5-hydroxymethylfurfural, comprising the following steps:
[0071] The compound containing metal ions and the ligand are mixed, and a hydrothermal synthesis reaction is carried out to obtain a catalyst for catalyzing the synthesis of 5-hydroxymethylfurfural from sugar.
[0072] In some specific embodiments of the present invention, the ligand is selected from the first ligand; in other embodiments of the present invention, the ligand is selected from the first ligand and the second ligand, the first ligand The molar ratio of the first ligand to the second ligand is preferably 1:(0-4), more preferably 1:(0-2).
[0073] Wherein, the selection of the first ligand and the second ligand type is as the selection of the first ligand and the second ligand type in the catalyst for catalyzing the synthesis of 5-hydroxymethylfurfural from sugar as described ...
Embodiment 1
[0092] Cobalt nitrate (23.3 mg, 0.08 mmol), tetrakis (4-phosphonophenyl) methane (25.6 mg, 0.04mmol), 2,2'-bipyridine (6.2mg, 0.04mmol), 15 ml of water were added to a 50 ml stainless steel reactor with a polytetrafluoroethylene liner, heated at 180°C for 3 days, After cooling to room temperature, blue rod-shaped crystals were obtained by centrifugation, washed with deionized water, dried in vacuum, and ground into fine particles with a mortar. The catalyst was named Co-MOF-11. Carry out infrared spectrum detection to catalyst, the result sees figure 1 , figure 1 Infrared spectrum of Co-MOF-11 prepared in Example 1.
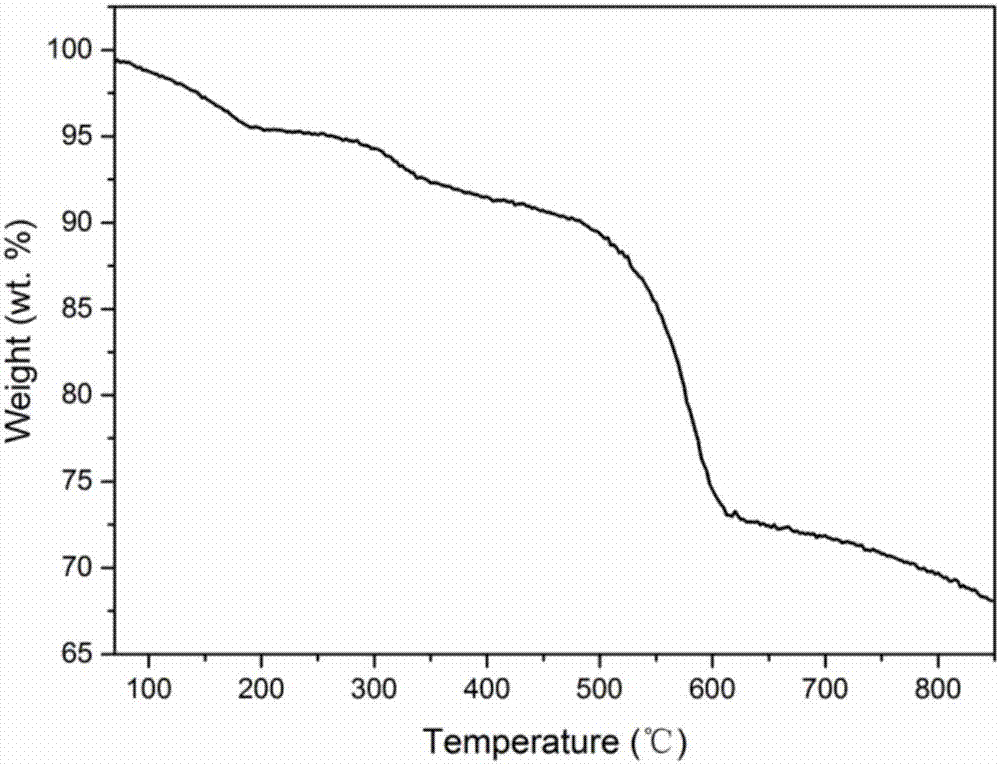
[0093] Carry out thermogravity detection to catalyst, the result sees figure 2 , figure 2 Thermogravimetric curve of Co-MOF-11 prepared for Example 1.
Embodiment 2
[0095] Cobalt nitrate (23.3 mg, 0.08 mmol), tetrakis (4-phosphonic acid phenyl) silane (26.3 mg, 0.04 mmol), 2,2'-bipyridine (6.2 mg, 0.04 mmol), 15 ml of water were added to a 50 ml stainless steel reactor with a polytetrafluoroethylene liner, heated at 180 ° C for 4 days, After cooling to room temperature, the blue rod-shaped crystals were obtained by centrifugation, washed with deionized water, dried in vacuum, and ground into fine particles with a mortar. The catalyst was named Co-MOF-21.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com