Reduction/pH double response adriamycin prodrug and preparation method and application thereof
A doxorubicin-responsive technology, applied in the field of biomedical polymer materials, can solve the problem of reducing the effect of drugs on cancer treatment, and achieve the effect of rapid synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
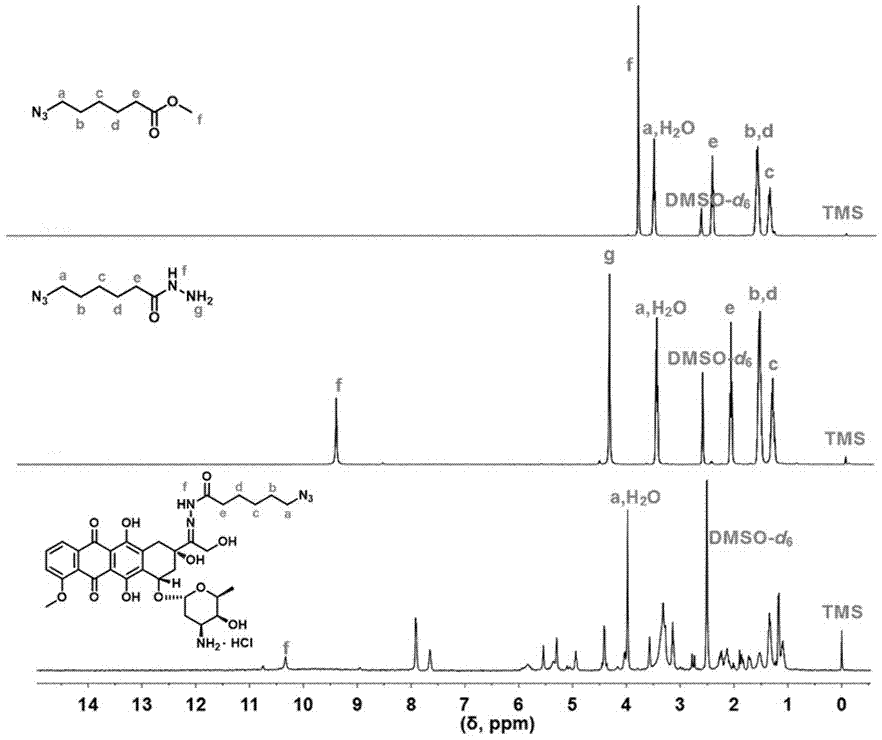
[0079] Embodiment one: doxorubicin derivative (N 3 - hyd -DOX×HCl) synthesis
[0080] Doxorubicin derivatives (N 3 - hyd -DOX×HCl) is mainly prepared through three steps. The first step is to prepare methyl 6-azidehexanoate; the second step is to prepare 6-azidehexanohydrazide; the third step is to modify doxorubicin hydrochloride (DOX×HCl) to obtain doxorubicin derivatives matter (N 3 - hyd -DOX×HCl), the specific synthesis method is as follows:
[0081] Synthesis of small molecular compound 6-azidohexanoic acid methyl ester. Take out the device previously placed in the oven at 120 °C, put it in a desiccator, wait for it to cool to room temperature, and take it out for use. Weigh 6-bromohexanoic acid methyl ester (3.54 g, 16.93 mmol) and sodium azide (NaN 3 ) (2.75 g, 42.33 mmol), and dissolved in 20 mL of DMF in a 50 mL round-bottomed flask, and reacted at 60 °C for 12 h under reflux of condensed water. Use neutral aluminum oxide (Al 2 o 3 ) short column to fi...
Embodiment 2
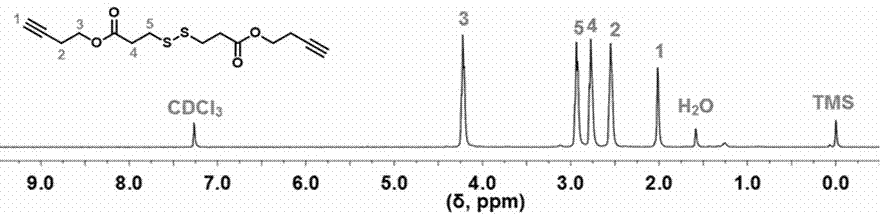
[0084] Example 2: Alkynyl-terminated 3,3'-dithiodiynbutyl dipropionate (B- ss -B) Synthesis of
[0085] First, 3,3'-dithiodipropionic acid and 3-butyn-1-ol were used as raw materials, and 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride was used as The activator uses 4-dimethylaminopyridine as a catalyst and dichloromethane as a solvent to obtain 3,3'-dithiodiynbutyl dipropionate through esterification. The specific synthesis method is as follows:
[0086]The glass instruments used were dried in an oven at 120 °C for 4 h, then taken out and cooled to room temperature in a desiccator for later use. First, add 3,3'-dithiodipropionic acid (3.66 g, 17.40 mmol), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride in sequence to the three-necked flask (EDC×HCl) (8.67 g, 45.23 mmol) and 4-dimethylaminopyridine (DMAP) (3.19 g, 2.61 mmol) in 100 mL of CH 2 Cl 2 dissolved; secondly, 3-butyn-1-ol (3.17 g, 45.23 mmol) and 15 mL of CH 2 Cl 2 Add to constant pressure...
Embodiment 3
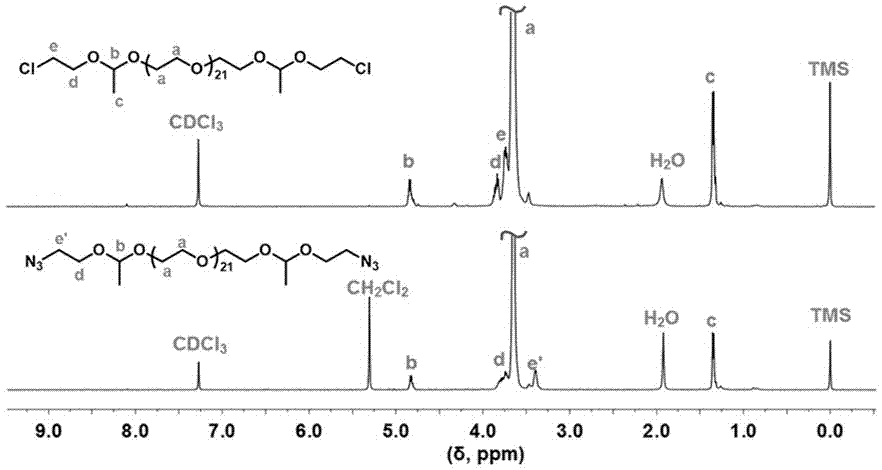
[0088] Embodiment three: diazide ethyl diacetal polyethylene glycol (N 3 - a -PEG 21 - a -N 3 )Synthesis
[0089] Azido-terminated compound diazide ethyl diacetal polyethylene glycol (N 3 - a -PEG 21 - a -N 3 ), obtained through a two-step reaction: First, the synthesis of dichloroethyl diacetal polyethylene glycol (Cl- a -PEG 21 - a -Cl); Secondly, the synthesis of diazide ethyl diacetal polyethylene glycol (N 3 - a -PEG 21 - a -N 3 ). The specific synthesis method is as follows:
[0090] The first step is to synthesize dichloroethyl diacetal polyethylene glycol (Cl- a -PEG 21 - a -Cl). Take polyethylene glycol (HO-PEG) with a molecular weight of 1000 22 -OH) (6.01 g, 6.01 mmol) and pyridinium p-toluenesulfonate (PPTS) (0.30 g, 1.20 mmol) were placed in a 100 mL branched flask, and 50 mL of toluene was added, and the toluene at normal pressure was used twice. The method of boiling removes impurity water. After it was cooled, under nitrogen protection, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com