Vaccination
A technique of vaccination, vaccination, applied in the field of elderly and immunocompromised human patients, which can solve the problem of unidentified immunological links
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0054] Preferred embodiments of the invention include:
[0055] - an immunogenic (e.g. vaccine) composition comprising the VZV gE antigen or derivative thereof truncated to remove the carboxy-terminal anchor region in combination with an adjuvant comprising QS21, 3D- MPL and liposomes comprising cholesterol for use in a method for protecting or preventing herpes zoster (HZ) in individuals 70 years of age or older for at least 5 years and / or postherpetic neuralgia.
[0056] - an immunogenic (e.g. vaccine) composition comprising the VZV gE antigen or derivative thereof truncated to remove the carboxy-terminal anchor region in combination with an adjuvant comprising QS21, 3D- MPL and liposomes comprising cholesterol, the composition for use in a method for protecting or preventing herpes zoster (HZ) and / or in individuals greater than 70 years of age for at least 5 years or postherpetic neuralgia.
[0057] - an immunogenic (e.g. vaccine) composition comprising the VZV gE antige...
Embodiment 1
[0070] Example 1 - Vaccine Efficacy Against HZ in Adults 50 Years and Older
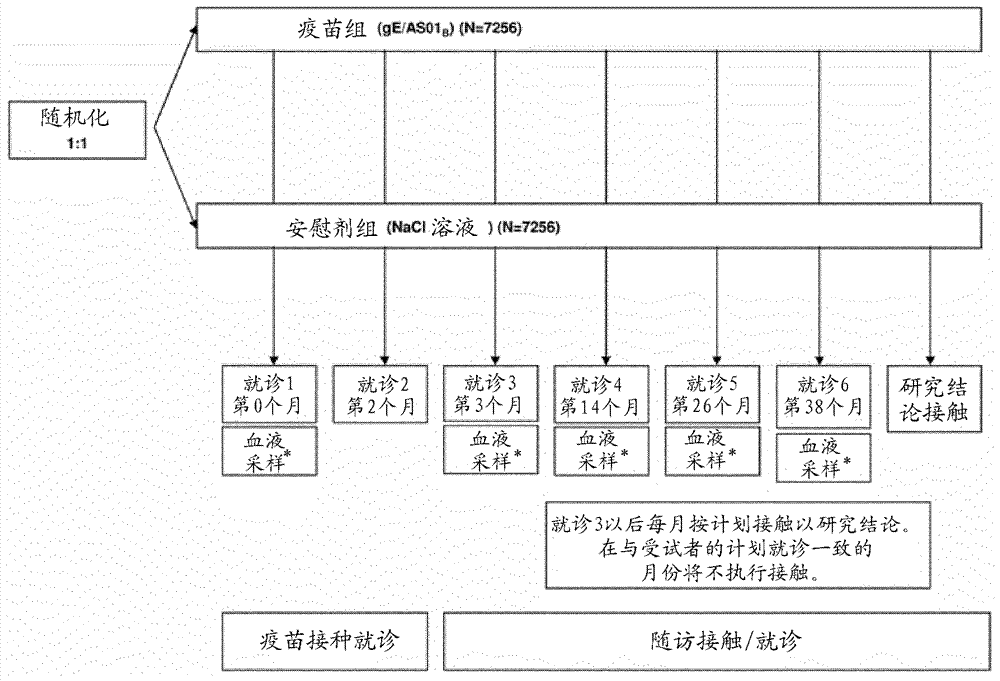
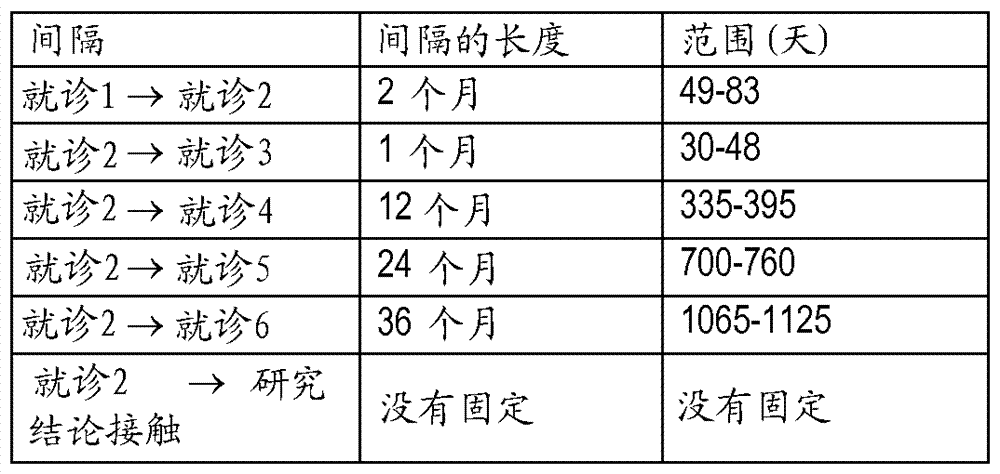
[0071] Example 1 describes the results of a Phase III, randomized, observer-blinded, placebo-controlled, multicentre, clinical vaccination trial that demonstrated Prophylactic efficacy, safety, and immunogenicity of a candidate HZ vaccine, GSK Biologicals' VZV gE / AS01B vaccine, when administered intramuscularly on a 2-month schedule.
[0072] The study population included men and women without severe immunocompromised conditions in the age ranges of 50-59 years (YOA), 60-69 YOA, 70-79 YOA, and >80 YOA. The 70–79 YOA layers and ≥80 YOA layers were pooled for primary analysis. Assigning approximately 20-25% of the ≥70 YOA group to those with ≥80 YOA ensures that this particularly susceptible population is adequately represented.
[0073] The candidate HZ vaccine tested in this trial was the adjuvanted recombinant VZV gE vaccine as described herein. Saline solution was included in this study as a neg...
Embodiment 2
[0097] Example 2 - Vaccine efficacy against HZ in immunocompromised adults
[0098] Example 2 describes a Phase III, randomized, observer-blinded, placebo-controlled, multicenter, clinical trial that demonstrated Prophylactic efficacy, safety and immunogenicity of an adjuvanted VZV gE vaccine candidate when administered intramuscularly in humans, here adult autologous hematopoietic stem cell transplantation (HCT) recipients.
[0099] The adjuvanted VZV gE vaccine candidate tested in this trial was the same vaccine candidate tested in the trial described in Example 1. Since the candidate vaccine is a subunit vaccine, there is no risk that the vaccine itself will cause varicella or HZ, a potential concern following vaccination with live VZV.
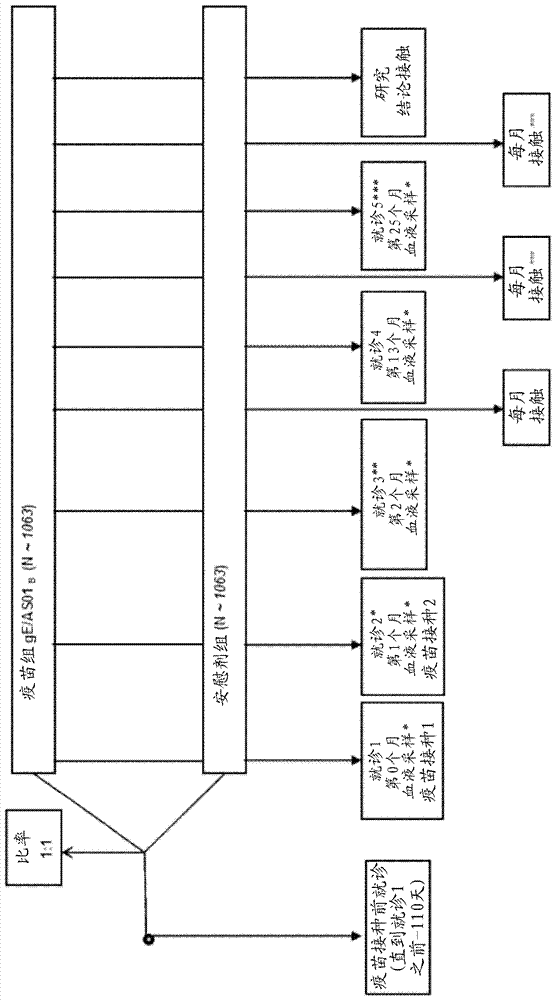
[0100] figure 2 The study design is depicted.
[0101] The primary aim of the clinical trial was to evaluate the vaccine efficacy (VE) for the prevention of HZ in autologous HCT recipients 18 years and older. Additional objectives incl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com